Leopold89
- 59
- 5
Dear community,
I have a question comparable to https://www.physicsforums.com/threads/calculating-the-mass-of-air-in-a-pressurised-air-tank.1053707/, but with some additional peculiarities.
My setup is:
1) I got a time series of gas pressure and temperature, with a time step of 2 minutes
2) I cannot measure the gas temperature, only the surrounding/room temperature
3) My gas can be either monoatomic or not and it has negligible contamination
4) The pressure is so large, that the gas turns into liquid
5) At arbitrary times during day time someone releases some gas from the tank, but not at night
Question: What is the remaining gas mass or particle number respectively?
My ansatz: First I can see that change in temperature will affect the pressure after some time delay, so I assume that should the tank contain a gas not at room temperature, the gas gets to room temperature after some time as well. This lag I can get from analysing the two time series.
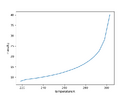
I would have assumed that I am moving along the p-T-curve when releasing gas, since some of the liquid evaporates until a new equilibrium between gas/vapour and liquid is reached, but the data shows I am well above this curve.
From this point onward I believe I am confusing myself. Now I know cannot use the ideal gas equation, because by releasing gas I am also converting liquid to gas/vapour, which should cool down the gas temperature below room temperature, followed by a heating phase, where the gas gets back to room temperature. As far as I understand I now need to somehow get the vapour volume, liquid volume, Gibbs energy and entropy to arrive at the remaining gas mass (or number of particles), right? I don't know how to get these.
I have a question comparable to https://www.physicsforums.com/threads/calculating-the-mass-of-air-in-a-pressurised-air-tank.1053707/, but with some additional peculiarities.
My setup is:
1) I got a time series of gas pressure and temperature, with a time step of 2 minutes
2) I cannot measure the gas temperature, only the surrounding/room temperature
3) My gas can be either monoatomic or not and it has negligible contamination
4) The pressure is so large, that the gas turns into liquid
5) At arbitrary times during day time someone releases some gas from the tank, but not at night
Question: What is the remaining gas mass or particle number respectively?
My ansatz: First I can see that change in temperature will affect the pressure after some time delay, so I assume that should the tank contain a gas not at room temperature, the gas gets to room temperature after some time as well. This lag I can get from analysing the two time series.
I would have assumed that I am moving along the p-T-curve when releasing gas, since some of the liquid evaporates until a new equilibrium between gas/vapour and liquid is reached, but the data shows I am well above this curve.
From this point onward I believe I am confusing myself. Now I know cannot use the ideal gas equation, because by releasing gas I am also converting liquid to gas/vapour, which should cool down the gas temperature below room temperature, followed by a heating phase, where the gas gets back to room temperature. As far as I understand I now need to somehow get the vapour volume, liquid volume, Gibbs energy and entropy to arrive at the remaining gas mass (or number of particles), right? I don't know how to get these.