Discussion Overview
The discussion revolves around calculating the mass of gas in a tank under specific conditions, including varying pressure and temperature, and the presence of both gas and liquid phases. Participants explore theoretical models and equations relevant to thermodynamics and fluid dynamics, while addressing complexities such as thermal inertia and phase changes.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
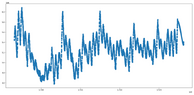
- One participant describes their setup involving time series data of gas pressure and temperature, noting the inability to measure gas temperature directly.
- Another participant questions whether thermal inertia and heat transfer rates are being considered in the calculations.
- Some participants suggest starting with simpler models, such as assuming adiabatic conditions or negligible thermal inertia, before tackling the full model.
- Equations involving pressure, temperature, and particle number are proposed, with some participants discussing the implications of phase changes on these calculations.
- There is mention of using Gibbs energy and internal energy equations to relate the properties of the gas and liquid phases, though uncertainty remains about how to proceed with these calculations.
- Participants express confusion regarding the integration of temperature and mass changes, and the relationship between enthalpy and entropy in the context of their equations.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the best approach to calculate the remaining gas mass or particle number. Multiple competing views and models are presented, with ongoing questions and challenges to various assumptions and methods.
Contextual Notes
Limitations include the dependence on specific assumptions about thermal inertia, phase behavior, and the accuracy of the models being discussed. Some participants express uncertainty about the applicability of certain equations given the presence of two phases.