alibaba2
- 31
- 0
hi to all,
this one may sound silly but i just can't seem to understand how it will work out.
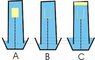
this is the setup - in a vertical water column (state of vacuum) a hollow box is attached to the base. all air from the box has been sucked out and the box contains a vacuum(fig A).
if the lid of the box is opened when inside the column, which of the following will take place?
- the vacuum in the box will automatically be taken by the surrounding water, in this way lowering the level of the water in the basin (fig B)
or
-the vacuum will release an ""empty space"" that will go to the top of the column and lead to the water level being raised (fig C)
10x
this one may sound silly but i just can't seem to understand how it will work out.
this is the setup - in a vertical water column (state of vacuum) a hollow box is attached to the base. all air from the box has been sucked out and the box contains a vacuum(fig A).
if the lid of the box is opened when inside the column, which of the following will take place?
- the vacuum in the box will automatically be taken by the surrounding water, in this way lowering the level of the water in the basin (fig B)
or
-the vacuum will release an ""empty space"" that will go to the top of the column and lead to the water level being raised (fig C)
10x