- #1
cathy
- 90
- 0
Homework Statement
http://img42.com/ASivg
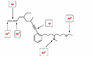
Please look at this image.
2. The attempt at a solution
Hello, I was looking at this image. Why is the top image sp? Shouldn't it be sp2 because there are double bonds on both sides? I understand the rest. Please advise, any help would be appreciated.
Thank you.
Attachments
Last edited by a moderator: