ken12380
- 1
- 0
Homework Statement
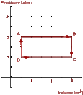
An ideal monatomic gas is confined to a cylindrical container having a movable, yet "snuggly-fitting," lid that slides up and down to accommodate changes in pressure and volume. The diagram shown below, outlines the steps in a heat cycle starting at position A and moving through positions B, C, D and then back to A.
*ignore the axes labels*
PV diagram2
Given:
PA = PB = 12.0 atm
PC = PD = 6.0 atm
VA = VD = 5.0 m3
VB = VC = 30.0 m3
temperature of point C is 900 K
moles of gas are present 2437.3376
How much total heat is added during the cycle?
Homework Equations
Q=nCp delta T
Q=nCv delta T
PV=nRT
The Attempt at a Solution
I tried:
Q=Q+Q=nCp delta T+nCv delta T=2437.3376*20.78*150+2437.3376*12.47*1500
=53187581 J but this is wrong.
Why? and what is the actual answer?