bertopolis
- 8
- 0
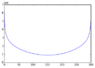
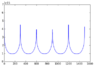
Hi I am currently trying to find the water absorption lines by using a Michelson interferometer, as a detector I am using an ocean spectrometer. The data obtained is thus the spectrum's "received" by the spectrometer. Am I right to assume that in order to find the absorption peak/peaks I should integrate the spectrum's obtained (between 700 and 1000nm), the plot of these would be a signal graph, which once this is Fourier transformed should give me the absorption lines? I am currently doing this using python and the results obtained are very misleading and unclear. The Fourier Transform's obtained will be attached to this post.
Thanks for any help
Thanks for any help