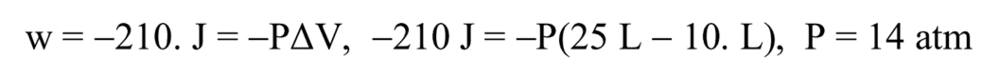SUMMARY
The discussion centers on the accuracy of a solution manual's approach to internal energy calculation, specifically regarding the omission of heat transfer. Participants confirm that the solution manual is correct in its context, as it serves to test understanding rather than provide a comprehensive analysis. The key equation referenced is work represented as PΔV, which highlights the relationship between work and energy without directly involving heat transfer. Additionally, there is a consensus that the manual incorrectly implies a direct conversion between work and heat energy, specifically the equivalence of 1 L atm to 1 J.
PREREQUISITES
- Understanding of thermodynamics principles, specifically internal energy and work.
- Familiarity with the equation PΔV in the context of work done by a system.
- Knowledge of the relationship between work and heat energy conversion.
- Basic comprehension of solution manuals and their purpose in educational contexts.
NEXT STEPS
- Research the principles of thermodynamics, focusing on internal energy and work.
- Study the implications of the PΔV equation in various thermodynamic processes.
- Explore the relationship between work and heat energy, including the laws of thermodynamics.
- Examine common misconceptions in solution manuals regarding energy conversions.
USEFUL FOR
Students and educators in thermodynamics, engineers dealing with energy calculations, and anyone seeking to clarify the concepts of work and heat transfer in energy systems.