etotheipi
I've come across some conflicting definitions and hoped someone more in-the-know could clarify a few points.
The rest energy is the total energy of the system in its rest frame; I read about the following (approximate!) semi-empirical mass formula earlier, and if we multiplied this through by ##c^{2}## we'd end up with the total rest energy of the nucleus equalling the sum of the inherent energy of the actual protons and neutrons, ##c^{2}[(A-Z)m_{n} + Zm_{p}]##, added to the other terms (which are all defined on Wikipedia, including contributions from the strong force etc.).
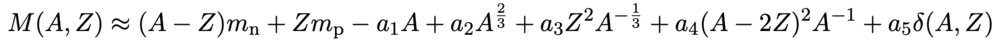
I'm wondering how the internal energy is defined generally (assuming it even is) in relation to the rest energy. In thermodynamics, it seems like it's taken to be the sum of kinetic energies relative to the COM and internal potential energies. However, this doesn't include the inherent mass-energies of the actual particles in your system, which led me to believe that the rest energy includes the internal energy.
I'm probably being naïve here, but how far off would I be in claiming that we could say the rest energy equals the sum of the internal energy and the mass-energies of the individual particles? In the context of the SEMF, I would be claiming that the first two terms can be considered the inherent mass-energy (##/c^{2}##) and the final five can be grouped into "internal energy" (##/c^{2}##). In this case, we'd always have ##\Delta E_{rest} = \Delta U##, which on a side-note also seems to fit nicely with the work-energy principle for non-rigid bodies.
Please do let me know if I'm sort of going in the right direction or if I'm still a few parsecs off...
The rest energy is the total energy of the system in its rest frame; I read about the following (approximate!) semi-empirical mass formula earlier, and if we multiplied this through by ##c^{2}## we'd end up with the total rest energy of the nucleus equalling the sum of the inherent energy of the actual protons and neutrons, ##c^{2}[(A-Z)m_{n} + Zm_{p}]##, added to the other terms (which are all defined on Wikipedia, including contributions from the strong force etc.).
I'm wondering how the internal energy is defined generally (assuming it even is) in relation to the rest energy. In thermodynamics, it seems like it's taken to be the sum of kinetic energies relative to the COM and internal potential energies. However, this doesn't include the inherent mass-energies of the actual particles in your system, which led me to believe that the rest energy includes the internal energy.
I'm probably being naïve here, but how far off would I be in claiming that we could say the rest energy equals the sum of the internal energy and the mass-energies of the individual particles? In the context of the SEMF, I would be claiming that the first two terms can be considered the inherent mass-energy (##/c^{2}##) and the final five can be grouped into "internal energy" (##/c^{2}##). In this case, we'd always have ##\Delta E_{rest} = \Delta U##, which on a side-note also seems to fit nicely with the work-energy principle for non-rigid bodies.
Please do let me know if I'm sort of going in the right direction or if I'm still a few parsecs off...