mishima
- 576
- 43
Hi, recently I've been playing with a little CO2 sensor. It is capable of reading ppm (parts per million) of CO2 from near the sensor up to a max of 10,000 ppm. We have a small apparatus for holding various absorbent chemicals, such as lithium hydroxide, and are controlling the release of CO2 from a small tank with a valve. Readings are tabulated automatically from a small micro-controller every 2 seconds, and later this information if plotted in google sheets. Here is an example:
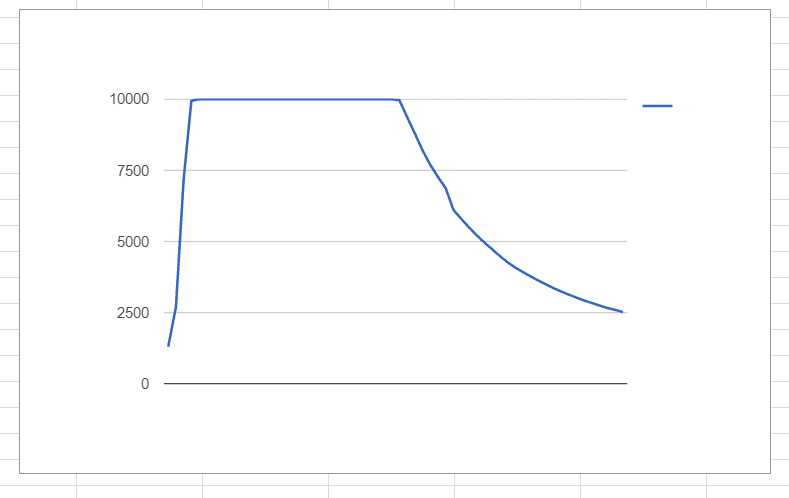
Unfortunately, the sensor's capabilities are beyond our current experimental setup. With even the smallest burst from our supply tank (roughly 60 mL of gas as standard temperature/pressure), the sensor quickly plateaus to its maximum before eventually settling down.again.
I was wondering if there was any mathematical technique able to interpolate/estimate the maximum value from the graphs we've been generating. The value does not need to be exact, even 20% accuracy would be fine for our purposes.
Unfortunately, the sensor's capabilities are beyond our current experimental setup. With even the smallest burst from our supply tank (roughly 60 mL of gas as standard temperature/pressure), the sensor quickly plateaus to its maximum before eventually settling down.again.
I was wondering if there was any mathematical technique able to interpolate/estimate the maximum value from the graphs we've been generating. The value does not need to be exact, even 20% accuracy would be fine for our purposes.