- #1
JeweliaHeart
- 68
- 0
[Note: Thread moved to homework forum by mentor]
This is one of my homework problems:
You are designing a counter flow heat exchanger for cooling a KOH electrolyte pH (11) in an electrolysis unit. The liquid is saturated with hydrogen. One of your team members suggests using copper pipes for good heat transfer. Is this a good idea; explain? Hint the dashed lines represent the hydrogen and oxygen evolution potentials for water as a function of pH.
The Pourbaix diagram is here.
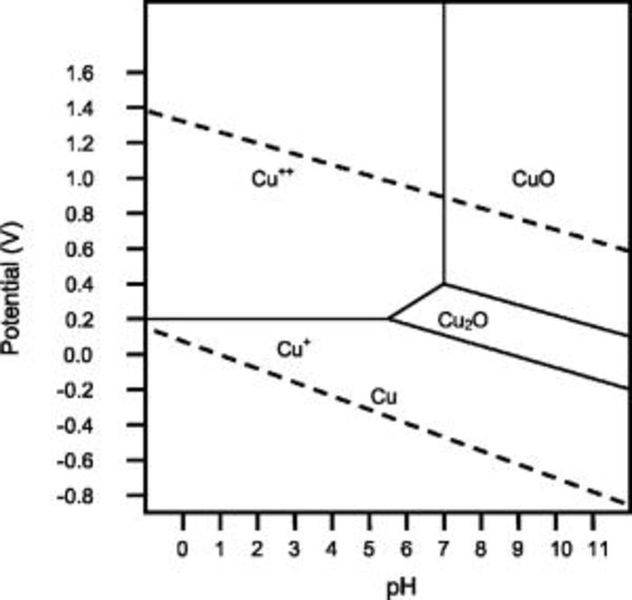
My Answer: For a solution saturated with hydrogen and in the alkaline region of the Pourbaix diagram, copper is a solid in a region of stability. Therefore, copper pipe is a good idea as there will be no oxidation of the copper pipe to CuO2 and copper ions will not be entering the KOH solution.I don't know if thsi answer is correct though. Some of my classmates said they thought it would corrode to Cu2O, but I thought we were supposed to use the bottom dashed line because it's in equilibrium with hydrogen. Thoughts please?
This is one of my homework problems:
You are designing a counter flow heat exchanger for cooling a KOH electrolyte pH (11) in an electrolysis unit. The liquid is saturated with hydrogen. One of your team members suggests using copper pipes for good heat transfer. Is this a good idea; explain? Hint the dashed lines represent the hydrogen and oxygen evolution potentials for water as a function of pH.
The Pourbaix diagram is here.
My Answer: For a solution saturated with hydrogen and in the alkaline region of the Pourbaix diagram, copper is a solid in a region of stability. Therefore, copper pipe is a good idea as there will be no oxidation of the copper pipe to CuO2 and copper ions will not be entering the KOH solution.I don't know if thsi answer is correct though. Some of my classmates said they thought it would corrode to Cu2O, but I thought we were supposed to use the bottom dashed line because it's in equilibrium with hydrogen. Thoughts please?
Last edited by a moderator: