JBD
- 15
- 1
I would like to clarify this particular question.
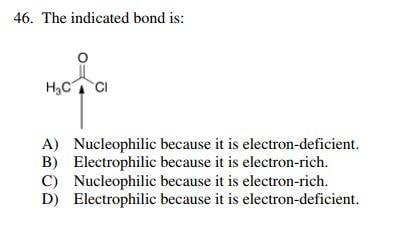
According to the answer key, the answer is D.

However, I argued to my friend that there may be an error in this question. The arrow is pointing toward a carbon atom so the question might have been " The indicated carbon atom is:". But if you take the question as it is, I answered C since I think that the "indicated bond" is the double bond and therefore nucleophilic and electron-rich. If it is not the double bond then what is the indicated bond in this question? And is it possible for a single bond to be electrophilic or nucleophilic? Thank you very much.
According to the answer key, the answer is D.
However, I argued to my friend that there may be an error in this question. The arrow is pointing toward a carbon atom so the question might have been " The indicated carbon atom is:". But if you take the question as it is, I answered C since I think that the "indicated bond" is the double bond and therefore nucleophilic and electron-rich. If it is not the double bond then what is the indicated bond in this question? And is it possible for a single bond to be electrophilic or nucleophilic? Thank you very much.