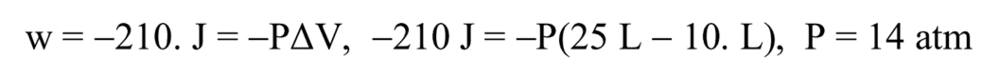Discussion Overview
The discussion revolves around the accuracy of a solution manual's approach to calculating internal energy, specifically questioning the absence of heat transfer in the solution. Participants explore the implications of this omission and the relationship between work and heat in thermodynamic contexts.
Discussion Character
- Debate/contested
- Technical explanation
- Conceptual clarification
Main Points Raised
- Some participants assert that the solution manual is correct, arguing that the omission of heat transfer is intentional to test understanding.
- Others express confusion about the reasoning behind the absence of heat transfer, seeking clarification on its relevance to the problem.
- One participant emphasizes that work is defined as ##P\Delta V## and questions the connection to heat transfer, suggesting that sign errors in equations may have led to misunderstandings.
- Another participant challenges the notion that work cannot be converted to heat, indicating a need for further discussion on this point.
- A participant critiques the solution manual for implying that 1 L atm equals 1 J, although they acknowledge that the final answer is correct.
Areas of Agreement / Disagreement
Participants exhibit disagreement regarding the correctness of the solution manual and the interpretation of work and heat transfer. Multiple competing views remain, with no consensus reached on the implications of the solution manual's approach.
Contextual Notes
Some participants express uncertainty about the definitions and relationships between work and heat, and there are unresolved questions regarding the mathematical steps involved in the calculations.