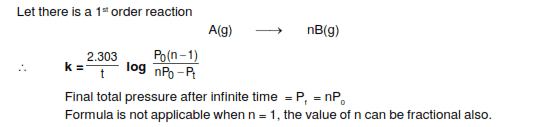SUMMARY
The discussion centers on the derivation of pressure kinetics in the context of first-order reactions, specifically under isothermal conditions. Participants clarify that for a reaction where one molecule of A produces one molecule of B, there is no change in pressure over time. The formula referenced relates to the pressure at the start (P0) and its behavior as time approaches infinity (t = ∞). The conversation emphasizes the simplicity of the kinetics involved, dismissing the need for complex derivations.
PREREQUISITES
- Understanding of first-order reaction kinetics
- Familiarity with isothermal reaction conditions
- Basic knowledge of chemical reaction equations
- Concept of pressure changes in chemical reactions
NEXT STEPS
- Research the derivation of first-order reaction kinetics
- Study isothermal reaction principles in physical chemistry
- Explore the relationship between pressure and concentration in reactions
- Learn about the implications of reaction stoichiometry on pressure changes
USEFUL FOR
Chemistry students, physical chemists, and professionals involved in reaction kinetics and thermodynamics will benefit from this discussion.