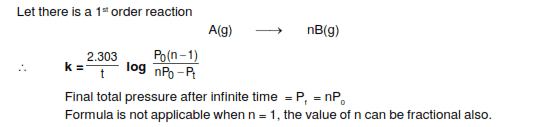Discussion Overview
The discussion revolves around the derivation and explanation of a formula related to kinetics and pressure in the context of physical chemistry. Participants explore the implications of pressure changes during chemical reactions, particularly focusing on isothermal reactions.
Discussion Character
- Exploratory, Technical explanation, Debate/contested
Main Points Raised
- One participant seeks clarification on a formula encountered in a physical chemistry book and requests a derivation.
- Another participant suggests looking up first-order reaction rates as a potential starting point for understanding the formula.
- A participant proposes that the discussion may pertain to isothermal reactions and questions the pressure at infinite time for a specific case where n = 1.
- One participant expresses frustration over their inability to derive the formula, questioning their understanding.
- Another participant suggests that the issue may stem from overcomplicating the problem, noting that in a simple reaction where one molecule of A produces one of B, there would be no change in pressure.
Areas of Agreement / Disagreement
Participants do not reach a consensus on the derivation or the implications of the formula. Multiple viewpoints are presented regarding the nature of the reaction and the associated pressure changes.
Contextual Notes
There are limitations in the discussion regarding assumptions about the reaction conditions, such as whether the reaction is indeed isothermal and the specific nature of the reactions being considered.