Paul Black
- 28
- 0
Hello everyone
I am trying to solve the following question from the book Analytical Chemistry 7th edition by Gard D. Christian - Page 164 Example 5.17
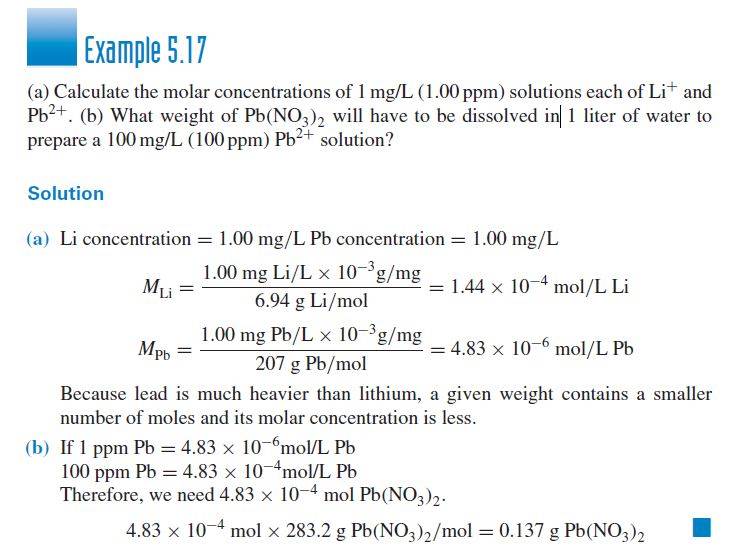
My problem is when I calculate the molecular weight of Pb(NO_3)_2 I get 331.2 g/mol but in the book they get 283.2 g/mol . Could you explain to me where they get this number. So instead of getting 0.137 g as last result, I get 0.16 g.
Also googling the question always gets me the same solution.
I am trying to solve the following question from the book Analytical Chemistry 7th edition by Gard D. Christian - Page 164 Example 5.17
My problem is when I calculate the molecular weight of Pb(NO_3)_2 I get 331.2 g/mol but in the book they get 283.2 g/mol . Could you explain to me where they get this number. So instead of getting 0.137 g as last result, I get 0.16 g.
Also googling the question always gets me the same solution.