- #1
Mohankpvk
- 102
- 3
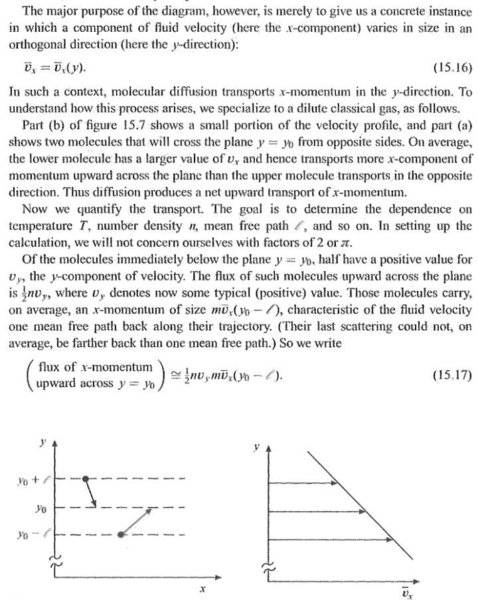
I was trying to understand the momentum transport between gas molecules in 2d.In the image below, it is stated that half of the molecules move up(positive velocity in y direction) and half negative.But the author didnt explain why he assumed it.