- #1
MigMRF
- 15
- 0
- Homework Statement
- A system contains 3 particles A, B and C. A can have the energies (0, Delta) while B and C can have the energies (0,Delta,6 Delta). Determine the partition function if the particles and distinguisable. Then determine the partition function if the particles are indistinguishable
- Relevant Equations
- Z=sum(e^(-beta*E))
I determined the partition function of the particle A, B and C.
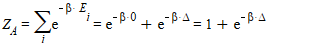
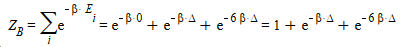
C should be the same as B.
I then considered the situation, where all particles are in the system at the same time, and drew a diagram of all possible arrangements:
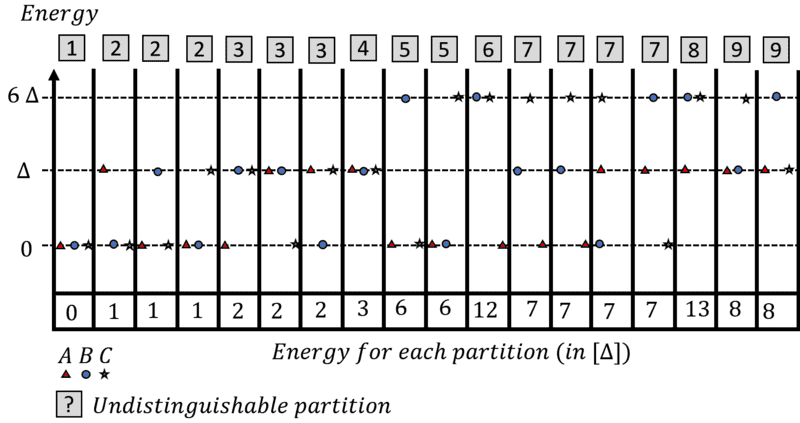
The grey boxes are the different partitions, given that we can't tell the difference on the particles. The number at the bottom of the table is the sum of all the energies.
From this table i created the two partition functions as shown below:
Distinguishable:
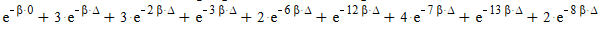
Indistinguishable:
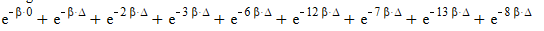
The correct answer is the following partition functions:
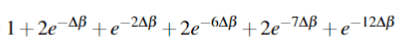
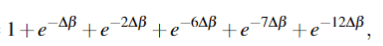
So my question is. Why does my method not work?
Kind Regards
C should be the same as B.
I then considered the situation, where all particles are in the system at the same time, and drew a diagram of all possible arrangements:
The grey boxes are the different partitions, given that we can't tell the difference on the particles. The number at the bottom of the table is the sum of all the energies.
From this table i created the two partition functions as shown below:
Distinguishable:
Indistinguishable:
The correct answer is the following partition functions:
So my question is. Why does my method not work?
Kind Regards