CFXMSC
- 33
- 0
Homework Statement
An electron of wavelength 1.74 \times 10^{-10} m strikes an atom of ionized helium (He+). What is the wavelength (m) of the light corresponding to the line in the emission spectrum with the smallest energy transition?
Homework Equations
Kinetic Energy
E_k=\frac{1}{2} \times m_e \times v_e^2
Photon Energy
E_p=\frac{h \times c}{\lambda_p}
Transition Energy
E_b=-k \times z^2 \times \left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right)
De Broglie wavelenght
v_e=\frac{h}{m_e \times \lambda_e}
Constants:
h=6.6260755 \times 10^{-34} J s
c=299792458 \ m/s
m_e=9.1093897 \times 10^{-31} \ kg
k=2.1789601284 \times 10^{-18}
Z_{He^+}=2
Problem constant:
\lambda_e=1.74 \times 10^{-10}
n_i=?
n_f=?
The Attempt at a Solution
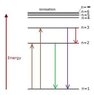
When the question say "smallest energy transition" what value of n_i=? and n_f=? should i use?
jumping this part and using energy balance i got that the kinetic energy of the electron must be equal to the transition energy plus photon energy:
\frac{1}{2} \times m_e \times v_e^2=-k \times z^2 \times \left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right)+\frac{h \times c}{\lambda_p}
\frac{1}{2} \times m_e \times \left(\frac{h}{m_e \times \lambda_e}\right)^2=-k \times z^2 \times \left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right)+\frac{h \times c}{\lambda_p}
Solving for \lambda_p
\lambda_p=\left[\frac{1}{2} \times m_e \times \left(\frac{h}{m_e \times \lambda_e}\right)^2 + k \times z^2 \times \left(\frac{1}{n_f^2}-\frac{1}{n_i^2}\right)\right]^{-1}\times h \times c
I've tryed for n_i and n_f 2 and 1, 3 and 2, 4 and 3 but without the right answer. What's wrong?