granpa
- 2,268
- 7
this is just an idea that I am presenting. it isn't established fact. the facts are here:
http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1392492&pageindex=8
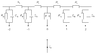
nodes 1234. nodes 3 and 4 and all further nodes are locked. meaning that they will not pass signals.
ap=action potential
t0 node 1 fires producing ap1 which moves at the speed of sound in water. 1500 m/s
t1 ap1 almost instantly reaches node 2 and passes through WITHOUT DELAY
t2 ap1 almost instantly reaches and ends at node 3 and unlocks node 3 (which takes some time)
t3 after 0.1 ms node 2 fires producing ap2 which moves at the speed of sound in water. 1500 m/s
t4 ap2 almost instantly reaches node 3 and passes through WITHOUT DELAY
t5 ap2 almost instantly reaches and ends at node 4 and unlocks node 4 (which takes some time)
t6 after 0.1 ms node3 fires producing ap3 which moves at the speed of sound in water. 1500 m/s
t0 node 1 fires producing ap1
t1 ap1 almost instantly reaches node 2
t1-t3 delay of 0.1 ms at node 2 before it fires
t3 node 2 fires producing ap2
t4 ap2 almost instantly reaches node 3
t4-t6 delay of 0.1 ms at node 3 before it fires
there is therefore only one delay and it is the 0.1 ms one that everyone already agrees on. so it takes 0.1 ms for an action potential at one node to create an action potential at the next node which is typically 1 or 2 mm away. that gives a net speed of 10-20 m/s. if there were no delay at each node then the signal would move at 1500 m/s. the speed of sound in water. (thats just a guess but its certainly at least a good fraction of that speed)
just before each node fires its ap it relocks itself so the ap can only go in one direction and the previous internode can immediately begin to return to its resting state. this is seen in curve C:
http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1392492&pageindex=8
http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1392492&pageindex=8
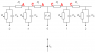
nodes 1234. nodes 3 and 4 and all further nodes are locked. meaning that they will not pass signals.
ap=action potential
t0 node 1 fires producing ap1 which moves at the speed of sound in water. 1500 m/s
t1 ap1 almost instantly reaches node 2 and passes through WITHOUT DELAY
t2 ap1 almost instantly reaches and ends at node 3 and unlocks node 3 (which takes some time)
t3 after 0.1 ms node 2 fires producing ap2 which moves at the speed of sound in water. 1500 m/s
t4 ap2 almost instantly reaches node 3 and passes through WITHOUT DELAY
t5 ap2 almost instantly reaches and ends at node 4 and unlocks node 4 (which takes some time)
t6 after 0.1 ms node3 fires producing ap3 which moves at the speed of sound in water. 1500 m/s
t0 node 1 fires producing ap1
t1 ap1 almost instantly reaches node 2
t1-t3 delay of 0.1 ms at node 2 before it fires
t3 node 2 fires producing ap2
t4 ap2 almost instantly reaches node 3
t4-t6 delay of 0.1 ms at node 3 before it fires
there is therefore only one delay and it is the 0.1 ms one that everyone already agrees on. so it takes 0.1 ms for an action potential at one node to create an action potential at the next node which is typically 1 or 2 mm away. that gives a net speed of 10-20 m/s. if there were no delay at each node then the signal would move at 1500 m/s. the speed of sound in water. (thats just a guess but its certainly at least a good fraction of that speed)
just before each node fires its ap it relocks itself so the ap can only go in one direction and the previous internode can immediately begin to return to its resting state. this is seen in curve C:
http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1392492&pageindex=8
Last edited: