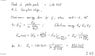vertices
- 62
- 0
Please look at the file problem.jpg. This is in reference to graph.jpg.
I'm trying to understand the solution (solutions.jpg).
I have a very trivial question. When a gamma ray photons enter the scintillator, it excites a electron, and a small fraction of the excitation energy is given out of visible/UV light during de-exitation. This is scintillation light.
It seems quite useless to me! I mean, what information does the scintillation light give us about the original photon? How on Earth can it give us information about the compton edge (when the energy transferred to the electron is a maximum).
I'm trying to understand the solution (solutions.jpg).
I have a very trivial question. When a gamma ray photons enter the scintillator, it excites a electron, and a small fraction of the excitation energy is given out of visible/UV light during de-exitation. This is scintillation light.
It seems quite useless to me! I mean, what information does the scintillation light give us about the original photon? How on Earth can it give us information about the compton edge (when the energy transferred to the electron is a maximum).