wishgames
- 2
- 0
I am attempting to use Eötvös rule
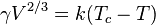 to find the surface tension of water but am not obtaining normal results.
to find the surface tension of water but am not obtaining normal results.
Surface Tension= 2.7*10^(-7)(374-x)*18^(-2/3)
at T=0 Celsius Surface tension = .0114 mN/m with this equation far from the normal 75.7 mN/m
what am I doing wrong?
Surface Tension= 2.7*10^(-7)(374-x)*18^(-2/3)
at T=0 Celsius Surface tension = .0114 mN/m with this equation far from the normal 75.7 mN/m
what am I doing wrong?
Last edited: