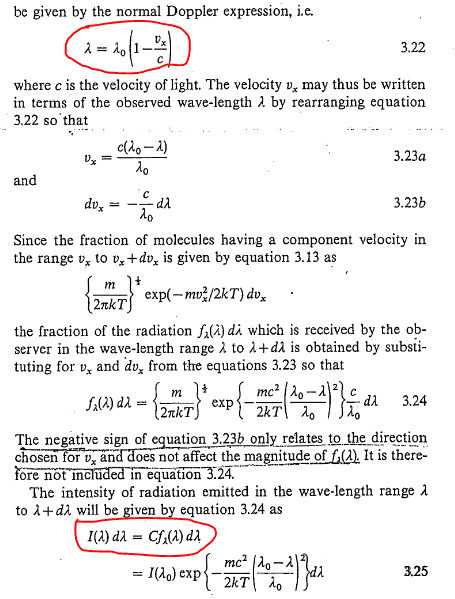
Homework Help Overview
The discussion revolves around understanding how to derive the spectral intensity formula using frequency, particularly in the context of the Doppler effect and statistical physics. Participants are exploring the relationship between frequency and intensity in a system involving moving atoms and photons.
Discussion Character
- Exploratory, Conceptual clarification, Assumption checking
Approaches and Questions Raised
- Participants are attempting to clarify the use of frequency in the context of the Doppler effect and how it relates to the intensity of emitted photons. There are discussions about the appropriate symbols for frequency and angular frequency, as well as the relevance of different statistical distributions.
Discussion Status
The discussion is ongoing, with participants providing initial guidance and questioning assumptions about the physics involved. Some participants are exploring the implications of the Doppler effect on frequency distribution, while others are addressing misunderstandings regarding the application of statistical mechanics.
Contextual Notes
There are mentions of specific equations and symbols that may lead to confusion, as well as a need to consider the classical nature of the system being discussed. The relevance of Bose-Einstein statistics is questioned, and participants are encouraged to revise fundamental concepts related to the Doppler effect and velocity distributions.