- #1
astrozilla
- 29
- 0
Homework Statement
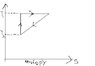
There is a temperature-Entropy graph (T-S) (attachment),which illustrates a hypothetical cyclic process.
a) Calculate the heat input or output along each of the paths.
b) Find an expression for the efficiency η of the complete cycle in terms of T1 and T2 only.
Homework Equations
for b ,η=(Q1/Q2)-1=(T1/T2 )-1,HEAT INPUT: DH=DQ (ONLY in case of isobaric change)