nazmus sakib
- 5
- 0
I have to derive
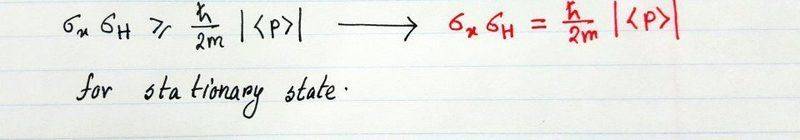
I did all the way but stuck with the ">=" to "=" sign. what is the logic behind it ? is it safe to write "approximate" while taking the square-root on both side ? or the energy term "V" has gone during the calculation so it has only momentum "p" ?
I did all the way but stuck with the ">=" to "=" sign. what is the logic behind it ? is it safe to write "approximate" while taking the square-root on both side ? or the energy term "V" has gone during the calculation so it has only momentum "p" ?