- #1
OmCheeto
Gold Member
- 2,389
- 2,946
I used to think I knew how batteries worked, but I've decided that if I did, I now don't.
Being that chemistry is one of my worst subjects, I decided to evade all of the anion, cation, this, that, and other terminology that I find incomprehensible and totally unable to devote to memory.
So here's my plan. I want to build a theoretical lead acid battery, using just single atoms.
I'm pretty sure that if I can understand this, I'll be back up to speed.
So here goes nothing.
Wiki lists the reactions at the negative and positive plates for discharge as follows:
So my negative plate will consist of a single neutrally charged lead atom and my solution will be a single hydrogen sulphate molecule with a net charge of minus one.
So now some hocus pocus happens and the lead atom and hydrogen sulphate molecule decide that they like each other, mate, and the progeny are lead sulphate, 3 positively charged hydrogen atoms, and 2 electrons.
And now some even more hocus pocus tomfoolery occurs when wiki states:
Where did all the electrons and ions run off to? And why do we now have sulphuric acid instead of hydrogen sulphate as our solution molecule? I understand that both sides of the reaction have to match, like an equation, as far as charge and total atoms go.
I guess [STRIKE]that is[/STRIKE] these are my [STRIKE]first[/STRIKE] third and fourth questions:
What is the liquid molecule in a charged battery? Hydrogen sulphate, or sulphuric acid?
Perhaps I should draw a picture...
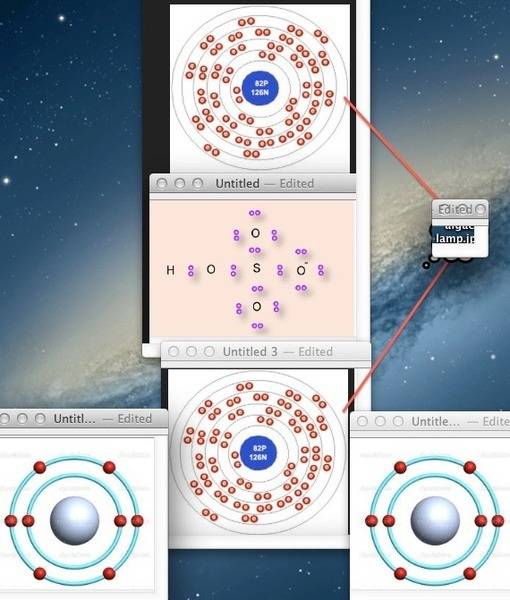
And where do the Hydrogen gas babies come from?

------------------------------------------------------------------------------------------
ps. I am not adverse to metaphor...
Om likes Evo.
Om is kind of neutral, but his outer shell is somewhat empty.
Evo has been referred to as "negative" by some, but Om and Evo are strangely attracted to each other.
They get married, become one, and have 3 hydrogen and 2 electron babies.
But who the hell is that guy over there? Why is he eating our babies? Why are the electron babies running through that lamp over there? And why is that 3rd guy pooping some weird watery lead sulphate spawn?

----------------------------
ok to delete, as it appears I've lost my mind...
Being that chemistry is one of my worst subjects, I decided to evade all of the anion, cation, this, that, and other terminology that I find incomprehensible and totally unable to devote to memory.
So here's my plan. I want to build a theoretical lead acid battery, using just single atoms.
I'm pretty sure that if I can understand this, I'll be back up to speed.
So here goes nothing.
Wiki lists the reactions at the negative and positive plates for discharge as follows:
Negative plate reaction: Pb(s) + HSO4-(aq) → PbSO4(s) + H+(aq) + 2-e
Positive plate reaction: PbO2(s) + HSO4-(aq) + 3H+(aq) + 2-e → PbSO4(s) + 2H2O(l)
So my negative plate will consist of a single neutrally charged lead atom and my solution will be a single hydrogen sulphate molecule with a net charge of minus one.
So now some hocus pocus happens and the lead atom and hydrogen sulphate molecule decide that they like each other, mate, and the progeny are lead sulphate, 3 positively charged hydrogen atoms, and 2 electrons.
And now some even more hocus pocus tomfoolery occurs when wiki states:
The total reaction can be written:
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(l)
Where did all the electrons and ions run off to? And why do we now have sulphuric acid instead of hydrogen sulphate as our solution molecule? I understand that both sides of the reaction have to match, like an equation, as far as charge and total atoms go.
I guess [STRIKE]that is[/STRIKE] these are my [STRIKE]first[/STRIKE] third and fourth questions:
What is the liquid molecule in a charged battery? Hydrogen sulphate, or sulphuric acid?
Perhaps I should draw a picture...
And where do the Hydrogen gas babies come from?

------------------------------------------------------------------------------------------
ps. I am not adverse to metaphor...
Om likes Evo.
Om is kind of neutral, but his outer shell is somewhat empty.
Evo has been referred to as "negative" by some, but Om and Evo are strangely attracted to each other.
They get married, become one, and have 3 hydrogen and 2 electron babies.
But who the hell is that guy over there? Why is he eating our babies? Why are the electron babies running through that lamp over there? And why is that 3rd guy pooping some weird watery lead sulphate spawn?

----------------------------
ok to delete, as it appears I've lost my mind...
