pink diamond
- 3
- 0
why on ftir graph there are several peaks for the same compound but at different wavelengths?
what causes the different modes of vibration for the same compound (PO4), what causes the v1 ,v3,v4 for PO4... help me please
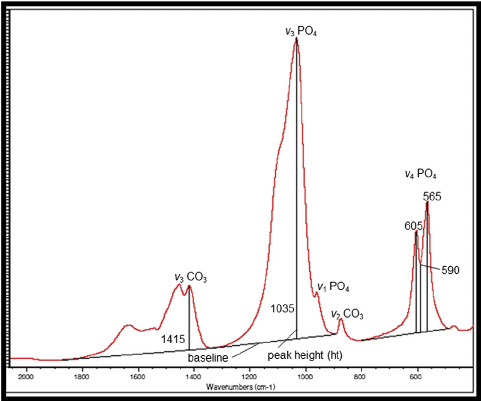
what causes the different modes of vibration for the same compound (PO4), what causes the v1 ,v3,v4 for PO4... help me please

