cr2504life
- 6
- 0
Hi and thanks for reading,
I don't really understand the valleys in the conduction band, in the E vs. k diagram, there is the L-valley, r-valley and X-valley. Each has a different momentum... and are at different energy levels.
I understand that at any temperature above absolute zero, a small fraction of electrons will acquire enough energy to jump to the conduction band (overcome the energy gap Eg).
The GaAs bandstructure here:
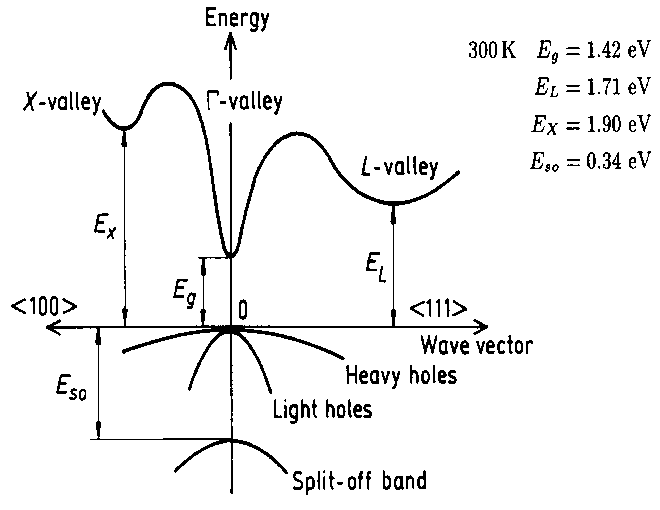
http://www.ioffe.ru/SVA/NSM/Semicond/GaAs/bandstr.html
I assume most electrons which acquire enough energy will jump to the lowest valley in the conduction band (r-valley for GaAs).
Ive been trying to determine what is the 'effective' temperature necessary to move an electron from the r-valley to the L-valley, the difference is 0.29 eV in GaAs.
Is the additional thermal energy comming from E = kT ?
... therefore T = 0.29eV*q/(k) ? this is a large temperature ... this seems too large ~3,600 °K.
How do the electons move between valleys ? And how does one find the required energy for this to take place ?
Thanks for any help you can provide.
I don't really understand the valleys in the conduction band, in the E vs. k diagram, there is the L-valley, r-valley and X-valley. Each has a different momentum... and are at different energy levels.
I understand that at any temperature above absolute zero, a small fraction of electrons will acquire enough energy to jump to the conduction band (overcome the energy gap Eg).
The GaAs bandstructure here:
http://www.ioffe.ru/SVA/NSM/Semicond/GaAs/bandstr.html
I assume most electrons which acquire enough energy will jump to the lowest valley in the conduction band (r-valley for GaAs).
Ive been trying to determine what is the 'effective' temperature necessary to move an electron from the r-valley to the L-valley, the difference is 0.29 eV in GaAs.
Is the additional thermal energy comming from E = kT ?
... therefore T = 0.29eV*q/(k) ? this is a large temperature ... this seems too large ~3,600 °K.
How do the electons move between valleys ? And how does one find the required energy for this to take place ?
Thanks for any help you can provide.
Last edited: