Urmi Roy
- 743
- 1
So I have a lot of trouble understanding what goes on along the triple line.
1. So within the dome, along the triple line, is the 'quality' defined anymore? If it is, what fraction of the pure substance is solid and what fraction is liquid?
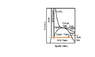
2.At the point on the extreme right end of the triple line, meeting the dome,is there solid and liquid or is it only vapor? (point C on the picture I've attached).
Similarly what phases exist at point A and B?
1. So within the dome, along the triple line, is the 'quality' defined anymore? If it is, what fraction of the pure substance is solid and what fraction is liquid?
2.At the point on the extreme right end of the triple line, meeting the dome,is there solid and liquid or is it only vapor? (point C on the picture I've attached).
Similarly what phases exist at point A and B?