Bolter
- 262
- 31
- Homework Statement
- Working out how to calculate binding energy
- Relevant Equations
- l = 1/2Nne
Here is the question:
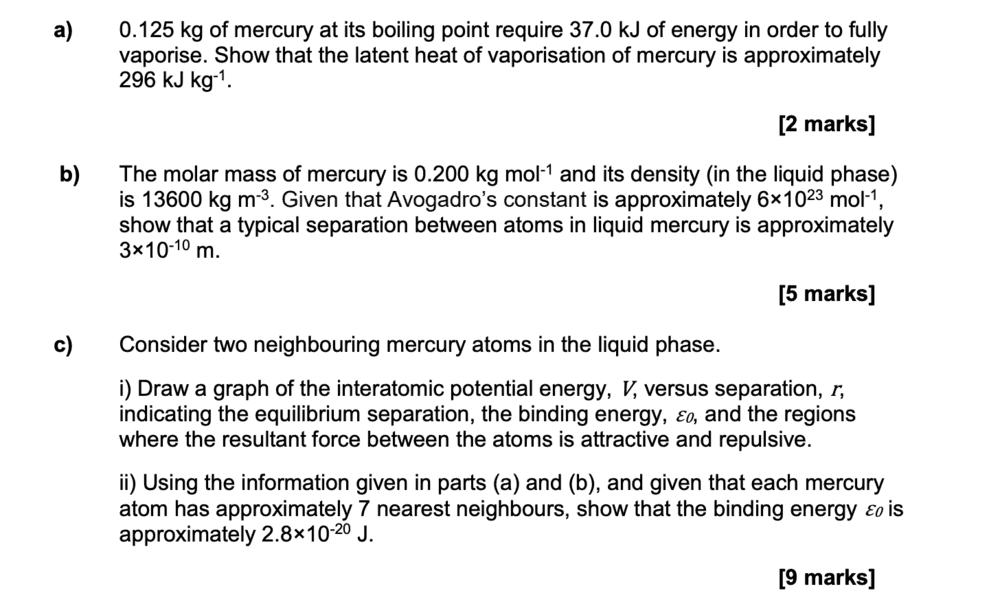
Stuck on how to complete part c)ii
Here is what I have done so far as well as trying to answer part c)ii

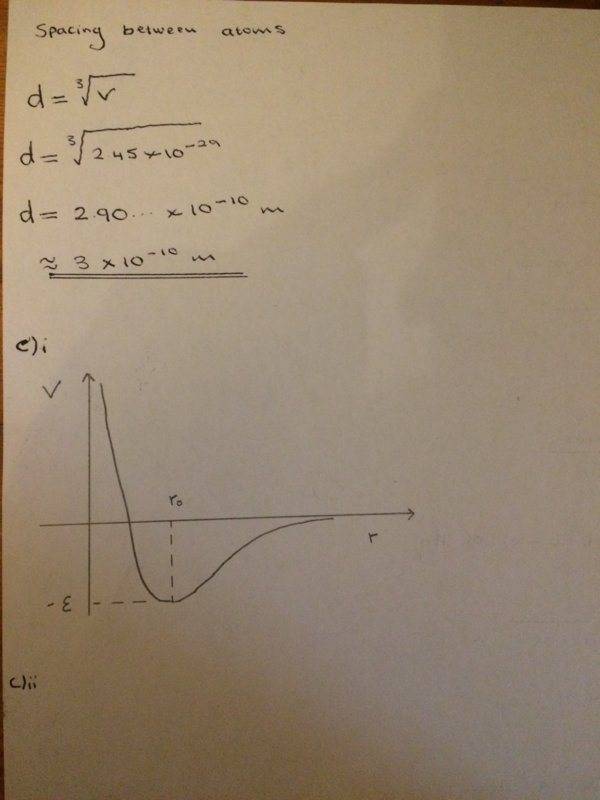
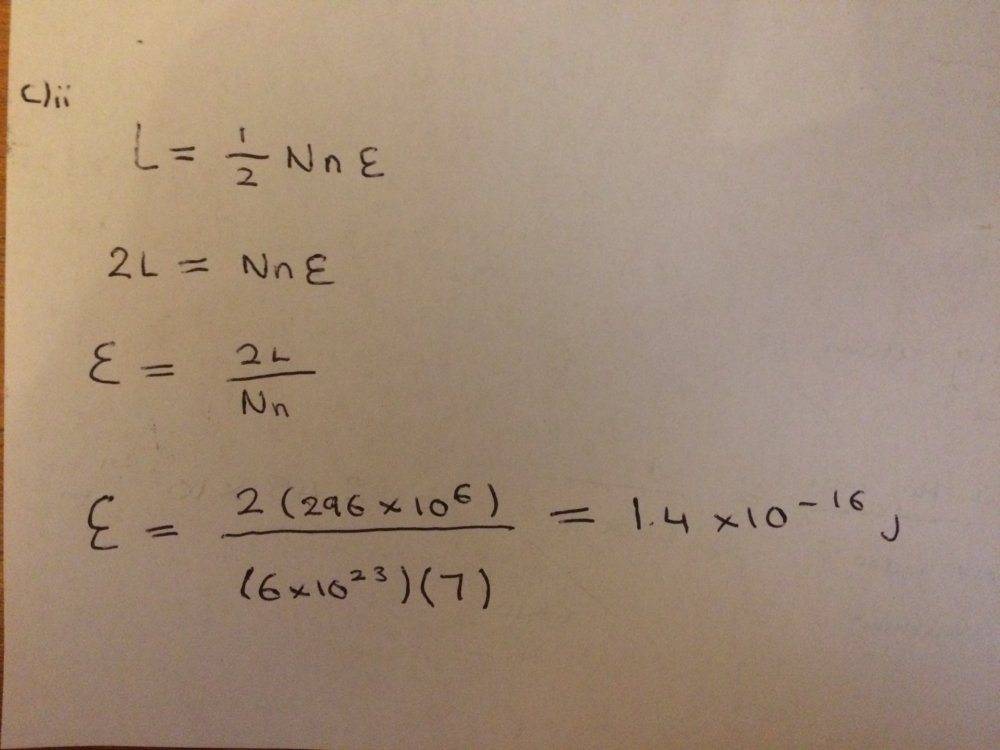
Any help would be appreciated! Thanks
Stuck on how to complete part c)ii
Here is what I have done so far as well as trying to answer part c)ii
Any help would be appreciated! Thanks
