silversurf
- 25
- 0
Homework Statement
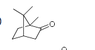
I attached a picture of the molecule. My question is why are the methyl group at the top not chemically equivalent. The answer says that this molecule makes three singlets in HNMR but I was thinking it should make only 2 because the top methyl groups are chemically equivalent aren't they?