SUMMARY
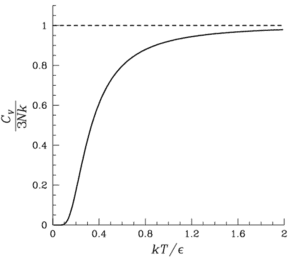
The heat capacity of solids drops exponentially at low temperatures due to the freezing of degrees of freedom, as explained by the Debye model. This phenomenon occurs because, at low temperatures, the vibrational modes of the solid's lattice structure become less active, leading to a significant reduction in heat capacity. The discussion emphasizes the importance of understanding the statistical mechanics behind this behavior, particularly the role of the Debye model in describing heat capacity at low temperatures.
PREREQUISITES
- Understanding of the Debye model for heat capacity
- Basic knowledge of statistical mechanics
- Familiarity with the concept of degrees of freedom in solids
- Awareness of heat capacity and its significance in thermodynamics
NEXT STEPS
- Research the mathematical derivation of the Debye model for heat capacity
- Explore the relationship between temperature and vibrational modes in solids
- Learn about the limitations of the Debye model at high temperatures
- Investigate experimental methods for measuring heat capacity in solids
USEFUL FOR
Students and researchers in physics, materials science, and engineering who are studying thermodynamic properties of solids and the behavior of heat capacity at varying temperatures.