BlackPowder
- 14
- 2
Helium has a higher 1st ionization energy (24.58eV) than N2 (15.6eV) and O2 (12.06eV). For an atmospheric room-temperature helium, why it is easier to get ionized than the daily life air under a same discharge setup? For example, for the Paschen curves, N2 locates at the left of He which means that for the same voltage and pressure, N2 requires us to move electrodes closer.
The most common answer I heard of this is that N2 is diatomic molecule which has more degrees of freedom than helium has. However, I don't think this can explain the following fact that N2 has a higher rate coefficient of electron impact ionization than He has, based on the calculation of the Boltzmann solver "BOLSIG+", even for a mean electron temperature (Te) much lower than the ionization thresholds of N2. Considering the electron temperature distribution function (EEDF) is near Maxwellian, if the mean Te is very low, the rate of N2 should higher.
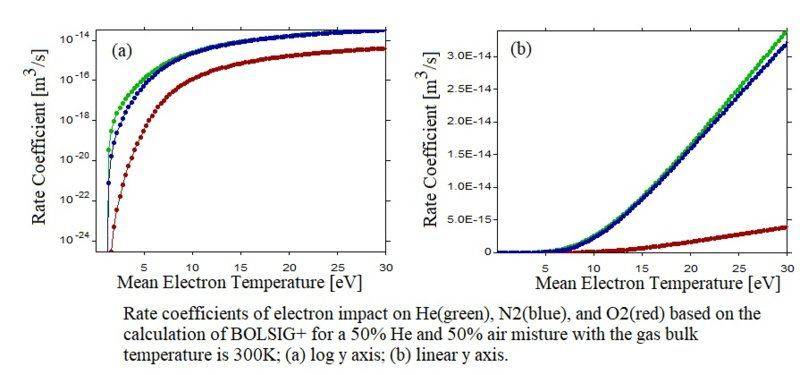
The most common answer I heard of this is that N2 is diatomic molecule which has more degrees of freedom than helium has. However, I don't think this can explain the following fact that N2 has a higher rate coefficient of electron impact ionization than He has, based on the calculation of the Boltzmann solver "BOLSIG+", even for a mean electron temperature (Te) much lower than the ionization thresholds of N2. Considering the electron temperature distribution function (EEDF) is near Maxwellian, if the mean Te is very low, the rate of N2 should higher.