ussername
- 60
- 2
I've learned that first thermodynamic law for some open system is in the form of:
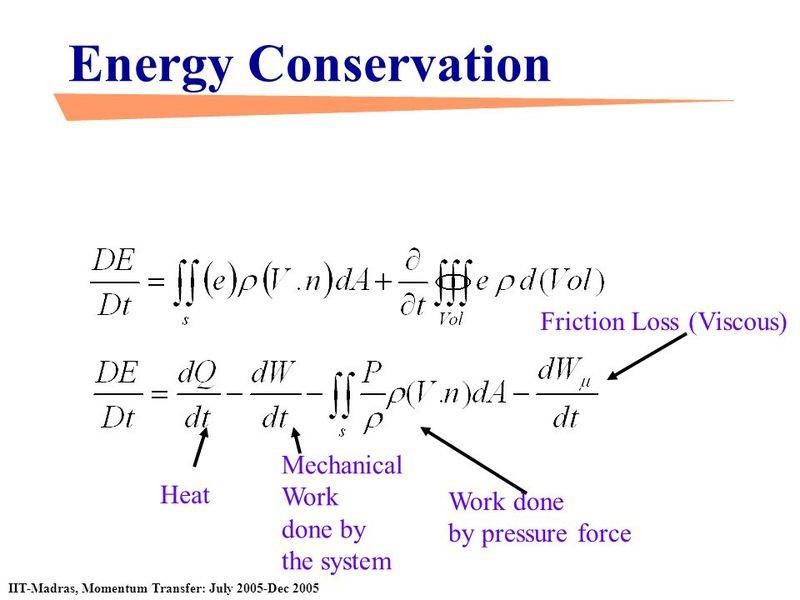
where total change of system energy ##\frac{\partial E}{\partial \tau }## is equal to the transferred heat and work.
Total change of system energy ##\frac{\partial E}{\partial \tau }## is equal to the energy transferred via mass flux (surface integral) plus energy change within the system (volume integral).
Now I've seen energy balance of CSTR reactor and it is in the form of:
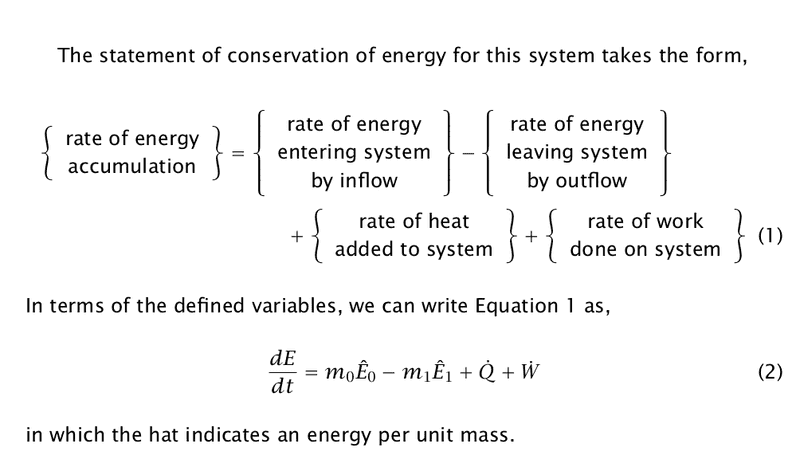
Full derivation is here or here.
I think ##\frac{\partial E}{\partial \tau }## in the bottom picture means total change of system (internal) energy, because further in the derivation it is replaced with total enthalpy differential and it is substituted with CSTR mass balance:
$$\frac{\partial n_{i}}{\partial \tau }=F_{i}^{0}-F_{i}+V\cdot \upsilon _{i}\cdot r_{V}$$
which makes sense only if ##\frac{\partial E}{\partial \tau }## is total change of energy.
If ##\frac{\partial E}{\partial \tau }## in the bottom picture means total change of system energy then it is not the same equation as in the first picture.
Can anybody explain?
where total change of system energy ##\frac{\partial E}{\partial \tau }## is equal to the transferred heat and work.
Total change of system energy ##\frac{\partial E}{\partial \tau }## is equal to the energy transferred via mass flux (surface integral) plus energy change within the system (volume integral).
Now I've seen energy balance of CSTR reactor and it is in the form of:
Full derivation is here or here.
I think ##\frac{\partial E}{\partial \tau }## in the bottom picture means total change of system (internal) energy, because further in the derivation it is replaced with total enthalpy differential and it is substituted with CSTR mass balance:
$$\frac{\partial n_{i}}{\partial \tau }=F_{i}^{0}-F_{i}+V\cdot \upsilon _{i}\cdot r_{V}$$
which makes sense only if ##\frac{\partial E}{\partial \tau }## is total change of energy.
If ##\frac{\partial E}{\partial \tau }## in the bottom picture means total change of system energy then it is not the same equation as in the first picture.
Can anybody explain?