- #1
Alex Hughes
- 54
- 13
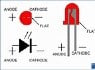
So, I'm new to electronics and I started to build some circuits with LEDs. I read up on how LEDs work and how they consist of a doped semiconductor material etc. But when I actually went to wire the LED in, it said the anode should be connected to the positive terminal of the power source. I'm confused, isn't the anode the source of electrons and the cathode is where the electrons flow? If that is true, shouldn't the anode be connected to the negative terminal? Also, if the anode is the source of the electrons, why is it referred to as the positive side almost everywhere I read? I would think if a bunch of electrons were accumulated there, it would be negatively charged. I'm really confused on this entire topic, would love somebody to clear things up. Thanks.