- #1
johnconnor
- 62
- 0
Guys I'm weak in heat and kinetic theory, so I'm going to need extra guide and pointers from you guys to solve this and the coming questions. Thank you.
Question:
The bulb of a constant-volume gas thermometer has a volume V and is connected by a long narrow capillary tube to a manometer, where there is a dead space of volume V/4 above the liquid. The thermometer is filled with an ideal gas at pressure p0 and temperature T0.
(i) Find an expression for the sensitivity of the thermometer (rate of change of pressure with temperature) at temperature T if the manomenter remains at temperature T0.
(ii) Sketch the variation of sensitivity with temperature for the range T/K = 0 to ∞.
(iii) Show, on the same axes, sensitivity as a function of temperature for the same bulb connected to a manometer with a dead space of negligible volume (a) when filled to give the same value of p0 at T0, and (b) when filled with the same mass of gas as previously.
Attempt:
(i) This is the diagram of the apparatus which I think is like. Is it correct?
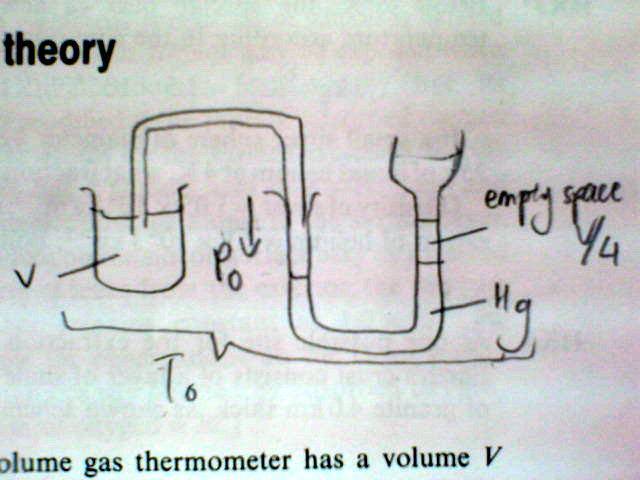
Or is it this one?
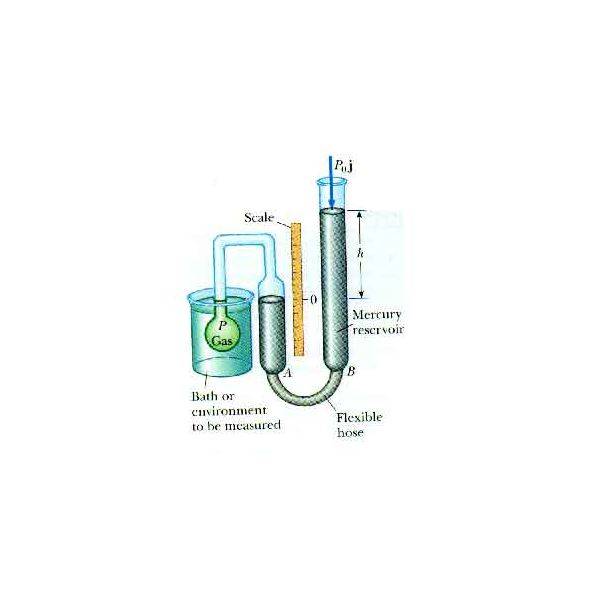
Question:
The bulb of a constant-volume gas thermometer has a volume V and is connected by a long narrow capillary tube to a manometer, where there is a dead space of volume V/4 above the liquid. The thermometer is filled with an ideal gas at pressure p0 and temperature T0.
(i) Find an expression for the sensitivity of the thermometer (rate of change of pressure with temperature) at temperature T if the manomenter remains at temperature T0.
(ii) Sketch the variation of sensitivity with temperature for the range T/K = 0 to ∞.
(iii) Show, on the same axes, sensitivity as a function of temperature for the same bulb connected to a manometer with a dead space of negligible volume (a) when filled to give the same value of p0 at T0, and (b) when filled with the same mass of gas as previously.
Attempt:
(i) This is the diagram of the apparatus which I think is like. Is it correct?
Or is it this one?