You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
What is Partition function: Definition and 213 Discussions
In physics, a partition function describes the statistical properties of a system in thermodynamic equilibrium. Partition functions are functions of the thermodynamic state variables, such as the temperature and volume. Most of the aggregate thermodynamic variables of the system, such as the total energy, free energy, entropy, and pressure, can be expressed in terms of the partition function or its derivatives. The partition function is dimensionless, it is a pure number.
Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the environment at fixed temperature, volume, and number of particles. The grand canonical partition function applies to a grand canonical ensemble, in which the system can exchange both heat and particles with the environment, at fixed temperature, volume, and chemical potential. Other types of partition functions can be defined for different circumstances; see partition function (mathematics) for generalizations. The partition function has many physical meanings, as discussed in Meaning and significance.
View More On Wikipedia.org
Each partition function is constructed to represent a particular statistical ensemble (which, in turn, corresponds to a particular free energy). The most common statistical ensembles have named partition functions. The canonical partition function applies to a canonical ensemble, in which the system is allowed to exchange heat with the environment at fixed temperature, volume, and number of particles. The grand canonical partition function applies to a grand canonical ensemble, in which the system can exchange both heat and particles with the environment, at fixed temperature, volume, and chemical potential. Other types of partition functions can be defined for different circumstances; see partition function (mathematics) for generalizations. The partition function has many physical meanings, as discussed in Meaning and significance.
View More On Wikipedia.org
-
A Similarity in form of time-evolution and Gibbs weight?
Why do the time-evolution operator in quantum mechanics ##\exp{iHt}## and the Gibbs-weight operator in statistical physics ##\exp{-H/T}## have the same functional form? – i.e. both exponentials of the Hamiltonian operator. The Matsubara trick/method just takes this as a fact in thermal QFT; but...- muscaria
- Thread
- Replies: 1
- Forum: Quantum Physics
-
G
I Usage of partition function in derivation of Sackur-Tetrode
Why do they introduce the partition function. I have seen it in the derivation of the Boltzmann distribution. But I don't know the physical significance of it here? And how do they get to (L.11) after that? I get everything until L.7. Including L.7. The rest of the proof is here just in case...- georg gill
- Thread
- Replies: 6
- Forum: Quantum Physics
-

Partition function of bound O2
Homework Statement Homework Equations I have question for (a) section. The Attempt at a Solution I have two answer for the question but I can't figure out which one is right. (1)Since the partition function is to sum up all the state in the system, I write down the answer (2)In other...- Kelly Lin
- Thread
- Replies: 8
- Forum: Introductory Physics Homework Help
-
M
I Symmetry on the partition function
We have a partition function ## \displaystyle Z=\frac{1}{N! \: h^{f}} \int dq\: dp \:e^{-\beta H(q,p)} ## And we obtain, for example, the pressure by ##\displaystyle p = \frac{1}{\beta} \frac{\partial\: \ln Z}{\partial V}##. So if we do the transformation ##Z \rightarrow a Z## where ##a >0##...- Mr rabbit
- Thread
- Replies: 2
- Forum: Classical Physics
-
J
Solve Problem: Partition Function for Magnetic Moment
Hi, maybe someone can help me with this problem? Homework Statement A system consist of N Atoms that have a magnetic moment m. The Hamiltonian in the presence of a magnetic field H is $$\mathcal{H}(p,q) - mH \sum_{i=1}^N cos(\alpha_{i})$$ where ##\alpha_i## is the angle between the magnetic...- Joe Cool
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-

Statistical Mechanics: Canonical Partition Function & Anharmonic Oscillator
Homework Statement With the Hamiltonian here: Compute the cananonical ensemble partition function given by ##\frac{1}{h} \int dq dp \exp^{-\beta(H(p,q)}## for 1-d , where ##h## is planks constant Homework EquationsThe Attempt at a Solution I am okay for the ##p^2/2m## term and the...- binbagsss
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
R
Partition function for ideal gas for "medium" temperature
Looking for the heat capacity of ideal gas due to rotational degrees of freedom. If the temperature of the gas is much higher than the temperature corresponding to the energy differential between states,the partition function can be written as the integral over the density of states. If the...- rockyleg
- Thread
- Replies: 1
- Forum: Introductory Physics Homework Help
-

A Classical gas with general dispersion relation
i'm trying to understand the solution to this problem: http://physweb.bgu.ac.il/COURSES/StatMechCohen/ExercisesPool/EXERCISES/ex_2065_sol_Y13.pdf (link to the problem and the solution of it) All my questions come from the partition function: 1) From where the term (2*pi)^d comes from?, I...- victor94
- Thread
- Replies: 1
- Forum: Classical Physics
-
T
Boltzmann Partition Function of H_2
Homework Statement In the real world, most oscillators are not perfectly harmonic. For a quantum oscillator, this means that the spacing between energy levels is not exactly uniform. The vibration levels of an ##H_2## molecule, for example, are more accurately described by the approximate...- transmini
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-

Statistical Mechanics -- partition function, change to polar coords
Homework Statement Hi I have the following definition for the partition function of ##N## particles in ##s## dimensions: I am looking at computing the partition function for this Hamiltonian: The solution is here: Homework Equations above The Attempt at a Solution I don't...- binbagsss
- Thread
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
S
Partition function for harmonic oscillators
Homework Statement Calculate the partition function, the entropy and the heat capacity of a system of N independent harmonic oscillators, with hamiltonian ##H = \sum_1^n(p_i^2+\omega^2q_i^2)## Homework Equations ##Z = \sum_E e^{-E/kT}## The Attempt at a Solution I am not really sure what to...- Silviu
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-

B Microcanonical vs. Canonical Partition Functions: What's the Difference?
What is the difference between micro canonical Partition function and canonical Partition function? Is the mathematical expression of the above two Partition function are same? If it is then why?? [emoji29]- soumobrata
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-
Q
Calculate Quantum Partition Function
Homework Statement Consider the case when the three Ising spins are replaced by quantum spins 1/2's with a Hamiltonian H=-J(s1.s2+s2.s3+s3.s1) calcualte the quantum partition function Homework Equations Partition function is the sum of E^(-H*B) where B is 1/kt The Attempt at a Solution...- QFT25
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
A The problematic 1/N in canonical partition function
The canonical partition function in classical statistical mechanics is calculated by ## Q_N(V,T)=\frac 1 {N! h^{3N}}\int e^{-\beta H(\mathbf q,\mathbf p)}d^{3N}q \ d^{3N}p ##. The ## \frac 1 {N!} ## is there to prevent the Gibbs paradox. But now consider a system of N particles that have no...- ShayanJ
- Thread
- Replies: 6
- Forum: Other Physics Topics
-

I Partition Function and Degeneracy
There doesn't seem to be a forum that is specifically about statistical mechanics, so I'm posting this question here. I apologize for the long-winded introduction, but I think it's needed to provide context for my question: If you have a discrete collection of single-particle energy levels...- stevendaryl
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
B
Two energy level system calculate average occupancy
Hi! I need help with the following question: A system has two energy levels, ε and ε1 that can be occupied by fermions (spin=1/2) that are non-interacting from a reservoir at temperature T and chemical potential μ. Compute the avarage occupation number of the state with energy ε. I have...- Bananen
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
D
Evaluating partition function (stuck for weeks)
Hi, I'm trying to calculate the partition function for a certain system and I arrived at an expression for the partition function $Z$, and have been stuck here for two weeks at the least. This is not a homework problem. If this is the wrong place to post a question like this, could you please...- dumbperson
- Thread
- Replies: 3
- Forum: Other Physics Topics
-
I
Partition function, Ideal gas, Entropy
Homework Statement For a diatomic gas near room temperature, the internal partion function is simply the rotational partition function multiplied by degeneracy ##Z_e## of the electronic ground state. Show that the entropy in this case is ## S = Nk\left[ \ln \left(...- Incand
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
S
A Partition function in quantum field theory
Why is the partition function ##Z[J]=\int\ \mathcal{D}\phi\ e^{iS[\phi]+i\int\ d^{4}x\ \phi(x)J(x)}## also called the generating function? Is the partition function a q-number or a c-number? Does it make sense to talk of a partition function in classical field theory, or can we define...- spaghetti3451
- Thread
- Replies: 6
- Forum: Quantum Physics
-
M
Partition function of electrons in a magnetic field
Hello everyone, How can I calculate the partition function of N classical electrons (forgetting about the spin) in a box of volume V with Hamiltonian (The Hamiltonian is missing a factor of 1/(2m)) ? I tried calculating the partition function of one electron first in the canonical ensemble but...- MMS
- Thread
- Replies: 1
- Forum: Electromagnetism
-

Canonical partition function for N ideal gasses
Homework Statement Exercise 4 in the upload titled Dok1.pdf. Write down an expression for the canonical partition function for N ideal Na2 gas molecules, when the rotational contribution is treated classically, and all inner degrees of freedom are treated quantum mechanically. Use this and...- Schwarzschild90
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I Partition Function: 2 Approaches & Questions Answered
I was reading about partition function. I noticed that there are two approaches toward partition function. The first approach: Suppose we are dealing with a closed system where the system is composed of heat bath (R) and inside it there is a very small system (E), the two systems are in thermal...- amjad-sh
- Thread
- Replies: 9
- Forum: Atomic and Condensed Matter
-
Z
Show interparticle spacing is much greater than wavelength
I have a past paper question from statistical physics: By assuming that ##\hbar^2 k^2=p^2##, I arrived at the result: The interparticle spacing, ##a^3 =\frac{V}{N}## is $$ a^3 >> e^{-\frac{p^2}{2mk_B T}} \lambda_{deB}^3$$ Is my assumption correct? and does the result complete the purpose of...- zeeshahmad
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
Partition function of harmonic oscillator with additional force
Homework Statement Show that the partition function for the harmonic oscillator with an additional force H = \hbar \omega a^{\dagger} a - F x_0 (a + a^{\dagger}) is given by \frac{e^{\beta \frac{F^2 x_{0}^2}{\hbar \omega}}}{1-e^{\beta \hbar \omega}} and calculate \left<x\right> = x_0...- brother toe
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-

I Finding the vibrational partition function of a diatomic molecule
Hi, How did they break down the following summation? When finding the vibrational partition function ofa diatomic molecule it was approximated that the energy levels of the vibrational part of the diatomic molecule were harmonic and therefore the energy equation for a harmonic oscillator was...- thegirl
- Thread
- Replies: 2
- Forum: Atomic and Condensed Matter
-

Statistical Mechanics, partition function for mixing
Hi! The following image is taken from my note in Stat Mech. Please excuse my ugly handwriting... I copied this from my professor's note on a whiteboard, and I'm not so sure if it is correct. The equations for Z1 (partition function before mixing) and Z2 (partition function after mixing) seems...- jin94
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
1
Partition Function for a helium atom
Homework Statement The first excited state of the helium atom lies at an energy 19.82 eV above the ground state. If this excited state is three-fold degenerate while the ground state is non-degenerate, find the relative populations of the first excited and the ground states for helium gas in...- 1v1Dota2RightMeow
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
1
Question on the degeneracies of a thermodynamic system
Homework Statement A system possesses three energy levels $$E_1=\varepsilon$$ $$E_2=2\varepsilon$$ $$E_3=3\varepsilon$$ with degeneracies $$g(E_1)=g(E_3)=1$$ $$g(E_2)=2$$. Find the heat capacity of the system. Homework Equations $$\beta=\frac{1}{kT}$$ $$Z=\sum_i g_ie^{-\beta \varepsilon_i} \...- 1v1Dota2RightMeow
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
M
Chem potential for adsorbed molecule from partition function
Homework Statement Consider a gas in equilibrium with a surface. The surface can adsorb the gas molecules onto any of M independent, distinguishable sites. The molecular partition function for an adsorbed molecule is q(T) ≡ exp[−β A_surface]. a) Assume that the adsorbed molecules are...- magnesium12
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
N
Example of Susy QM and its partition function
I am reading an article by Tachikawa on the Nekrasov partition function ("A review on instanton counting and W-algebras"). The article is meant to be pedagogical but I have some trouble with what is supposed to be "baby" examples. The first one involves susy QM on \mathbb{C}^2 . He says the...- nrqed
- Thread
- Replies: 9
- Forum: Beyond the Standard Models
-
Q
What is the Limit of the Partition Function in the Low Temperature Regime?
Homework Statement Ground state energy is set at 0. E_n=\left(1-\frac{1}{n+1}\right)\in with no degeneracy (\Omega(n)=1); (n=0,1,2...) Write down the partition function and look for its limit when kt \gg \in\\ kt \ll \in Homework EquationsThe Attempt at a Solution Partition function for this...- quanlop93
- Thread
- Replies: 5
- Forum: Advanced Physics Homework Help
-
Partition function and vacuum energy (basic stuff)
Hi all This is a fairly basic QFT question but it's bothering me. And Peskin/Schroeder fails at explaining basic stuff, so here I am. After calculating Z for a particular theory I know this can be used to calculate all kinds of correlation functions. Itself, however, is the probability...- diegzumillo
- Thread
- Replies: 6
- Forum: High Energy, Nuclear, Particle Physics
-
D
Average number of particles/subsystems in a state
Homework Statement A system in thermal equilibrium at temperature T consists of a large number of subsystems, each of which can exist only in two states of energy and , where . In the expressions that follow, k is the Boltzmann constant. For a system at temperature T, the average number of...- devd
- Thread
- Replies: 1
- Forum: Advanced Physics Homework Help
-
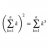
Microcanonical partition function - Dirac delta of operators
Homework Statement Why is it that the microcanonical partition function is ##W = Tr\{\delta(E - \hat{H})\}##? As in, for example, Mattis page 62? Moreover, what's the meaning of taking the Dirac delta of an operator like ##\hat{H}##? Homework Equations The density of states at fixed energy is...- ddd123
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
F
Ready to Explore the Wonders of Physics? Join the Conversation on PF!
Hi guys, New to the forum! Hope we have some fun with Physics in here! Cheers- Fotisd
- Thread
-
- Tags
- partition function
- Replies: 1
- Forum: New Member Introductions
-
F
Why Cannot I Factorize the Partition Function?
Hi everibody, the other day in a stadistical physics lesson we were studyng Fermi Dirac and Bose Einstein stadistics and comparing it to the classical Maxwell Boltzmann's. We learned that in the quantum stadistics for indistinguishable particles the partition function of the whole system...- Frank Einstein
- Thread
- Replies: 4
- Forum: Atomic and Condensed Matter
-
R
Saha equation partition function for Argon?
This question is in regards to the degeneracy of states for an Argon atom with just one missing electron. For hydrogen the problem of finding the partition function depends on finding the the ionized state of hydrogen divided by the non-ionized state... (please see Saha equation ->...- randombill
- Thread
- Replies: 5
- Forum: Astronomy and Astrophysics
-
A
Ising Model and Partition FUnction
Hi all This is probably a naïve question to ask, but I am puzzled by it and need an answer. The first time I encountered the term 'partition function' that was in context of Boltzmann distribution. But the same formulas of manipulating a partition function ( to obtain free energy, temperature...- A Dhingra
- Thread
- Replies: 1
- Forum: Other Physics Topics
-

Derive Internal Energy from Thermodynamic Identity
Homework Statement For a single molecule, derive the internal energy U = 3/2kBT In terms of the partition function Z, F = -kBTlnZ Where Z = V(aT)3/2 Homework Equations Thermodynamic identity: δF = -SδT - pδV p = kBT/V S = kB[ln(Z) + 3/2]The Attempt at a Solution U = F + TS δU = δF +...- SalfordPhysics
- Thread
- Replies: 2
- Forum: Advanced Physics Homework Help
-
M
Meaning of the Partition function
Hi all, I am struggling to grasp the sense of the partition function. First of all, I had a look at a couple of derivations (which the relevant Wikipedia page follows) in which the concept of heat"energy of a thermal bath" is invoked. Well this is already confusing me: if the thermal bath has an...- muzialis
- Thread
- Replies: 2
- Forum: Other Physics Topics
-
M
Partition Function Homework: Solve for N Particles in Spherical Container
Homework Statement An ideal monatomic gas at the temperature T is confined in a spherical container of radius R. There are N molecules of mass m in the gas. Molecules move in a spherically symmetric potential V(r) where r is the distance from the center of the container. The potential V(r) is...- muskie25
- Thread
- Replies: 8
- Forum: Advanced Physics Homework Help
-
N
Grand Canonical Partition function
Hi. 1) Does the Grand canonical partition function ##\Xi## behave in a similar fashion to the good old canonical partition function ##Z##? Do you calculate thermodynamical quantities (entropy, hemholtz free energy etc.) in similar fashions? 2) Is it possible for some kind soul here to...- Nikitin
- Thread
- Replies: 12
- Forum: Classical Physics
-
W
Partition Function for Positronium
First off, I'm glad I'm finally a member on this board. It has helped me TREMENDOUSLY over the past few years with various problems I've had. You guys/gals are awesome and hopefully I can make some contributions to this site. 1. Homework Statement A. Write down the partition function for...- wilsonaj4
- Thread
- Replies: 6
- Forum: Advanced Physics Homework Help
-
T
Generalising the Ising model to multiple spin values
My tutor asked us today to consider the partition function of the following model as an aside to our topic at the moment. I went to work out the maths of it today and I'm quite stuck for how the calculation can proceed. It's a 1d closed chain with some number, n, points. Each point has some...- TSRUser1234
- Thread
- Replies: 3
- Forum: Atomic and Condensed Matter
-
R
Hydrogen partition function and degeneracy
So I'm trying to use the excitation law of Boltzmann and the ionisation law of Saha to calculate stuff about what percentage of a quantity of hydrogen in ionised and in what energy state. I have the temperature and energy levels values, so i still need the: -statistical weight (degeneracy)...- Raezeman
- Thread
- Replies: 1
- Forum: Atomic and Condensed Matter
-
P
How to get rid of units in Partition Function
Hi guys, I'm studying a classical ideal gas trapped in a one-dimensional harmonic potential and I first want to write out the partition function for a single particle. This, I believe, requires two Gaussian integrations, like so: Z=\int_{-\infty}^{\infty} d\dot{x}...- "pi"mp
- Thread
- Replies: 4
- Forum: Classical Physics
-
C
Semi-Classical/Classical derivation of ideal gas partition function
In the semi-classical treatment of the ideal gas, we write the partition function for the system as $$Z = \frac{Z(1)^N}{N!}$$ where ##Z(1)## is the single particle partition function and ##N## is the number of particles. It is semi-classical in the sense that we consider the...- CAF123
- Thread
- Replies: 1
- Forum: Classical Physics
-
S
Imaginary Partition function
I have a partition function in euclidean quantum field theory. I have a parameter, let's say a charge, that I can change in the action that define the partition function. I found that for small charge the partition function is positive, but there is a critical charge, above the one the...- santale
- Thread
- Replies: 4
- Forum: Quantum Physics
-
H
Interpretation verification: Partition function vs. number of states
Greetings, I have been studying stat mech lately, and while I have gotten good at using partition functions to solve problems, I wanted to check my interpretation of what a partition function is, and especially to contrast it with the number of states. So, I'm just looking for a yes or no to...- HJ Farnsworth
- Thread
- Replies: 2
- Forum: Classical Physics
-
U
Partition function of simple system
Homework Statement A molecule has 4 states of energy -1, 0,0 and 1. Find its partition function and limit of energy as T → ∞. Homework Equations The Attempt at a Solution Z = \sum_r e^{-\beta E} = e^{-\beta} + 2 + e^{\beta} U = -\frac{\partial ln(Z)}{\partial \beta} = \frac{e^{-\beta} -...- unscientific
- Thread
- Replies: 4
- Forum: Advanced Physics Homework Help