- #1
[sammich]
- 4
- 0
Alright so I know the common answer:
Enantiomers - same chemical and physical properties
Diastereomers - different chemical and physical properties
In lecture, our professor said that enantiomers can have different biological effects. I was confused because, as he had said earlier, they have the same chemical properties. I did some research into it and found that enantiomers react differently with chiral molecules. Therefore because there is an exception to the rule, by definition of chemical property, enantiomers do not always have the same chemical properties.
That was 3 weeks ago.
Today we had an exam, and unexpectedly, there were 2 T/F questions exactly as follows:
___ Enantiomers have the same chemical and physical properties
___ Diastereomers have the same chemical and physical properties
I didn't even think about it and marked FALSE for both. Now I'm sitting here kicking myself because I know my professor is going to mark the first question wrong. I'm trying to get all my facts straight so when I get the test back, I can explain my reasoning to him, and that his question did not specify if the enantiomers were in achiral environments.
My logic is as below: (Sorry, could only think of it using discrete math notation: upsidedown A means "for all", backwards E means "there exists")
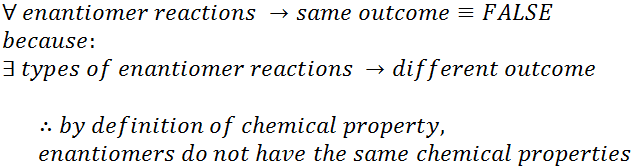
If I wasn't rushing to finish the exam in time, I would've been more thorough and remembered that there was a discrepancy between the lecture and what I had found, and I would have asked him to clarify the question. He's a very educated guy, and very approachable, so I will be able to explain my reasoning. But... before I make a fool of myself, I just want to make sure that I am not mistaken
So... Am I misinterpreting something?
Thanks in advance!
[Oh, and I also read that enantiomers can have different smells and tastes... Are those not physical properties? Then why is our textbook, like many online sources, saying they have identical physical properties?]
EDIT: nevermind the smell/taste question, that just brings me back to the original question because smell and taste are interactions with our bodies, which contain chiral compounds
Enantiomers - same chemical and physical properties
Diastereomers - different chemical and physical properties
In lecture, our professor said that enantiomers can have different biological effects. I was confused because, as he had said earlier, they have the same chemical properties. I did some research into it and found that enantiomers react differently with chiral molecules. Therefore because there is an exception to the rule, by definition of chemical property, enantiomers do not always have the same chemical properties.
That was 3 weeks ago.
Today we had an exam, and unexpectedly, there were 2 T/F questions exactly as follows:
___ Enantiomers have the same chemical and physical properties
___ Diastereomers have the same chemical and physical properties
I didn't even think about it and marked FALSE for both. Now I'm sitting here kicking myself because I know my professor is going to mark the first question wrong. I'm trying to get all my facts straight so when I get the test back, I can explain my reasoning to him, and that his question did not specify if the enantiomers were in achiral environments.
My logic is as below: (Sorry, could only think of it using discrete math notation: upsidedown A means "for all", backwards E means "there exists")
If I wasn't rushing to finish the exam in time, I would've been more thorough and remembered that there was a discrepancy between the lecture and what I had found, and I would have asked him to clarify the question. He's a very educated guy, and very approachable, so I will be able to explain my reasoning. But... before I make a fool of myself, I just want to make sure that I am not mistaken
So... Am I misinterpreting something?
Thanks in advance!
[Oh, and I also read that enantiomers can have different smells and tastes... Are those not physical properties? Then why is our textbook, like many online sources, saying they have identical physical properties?]
EDIT: nevermind the smell/taste question, that just brings me back to the original question because smell and taste are interactions with our bodies, which contain chiral compounds
Last edited: