- #1
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
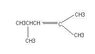
What is the structure of the polymer produced by this type of polymerization?
- Thread starter vg19
- Start date
In summary, the conversation involves a person seeking help with a question regarding the structure of a polymer produced by polymerization. The other person provides guidance and suggests redrawing the molecule to make it easier. They also confirm that the IUPAC name for the molecule is 2,4-dimethyl-2-pentene.
Chemistry news on Phys.org
- #2
ShawnD
Science Advisor
- 718
- 2
Redraw the molecule so that you can draw a straight horizontal line from each of the [itex]sp^2[/itex] carbons. From there it should be fairly straight forward.
- #3
- #4
ShawnD
Science Advisor
- 718
- 2
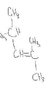
That's exactly what I was thinking. Now just break the double bond and start drawing the same thing over and over again. 
- #5
vg19
- 67
- 0
great thanks! just 1 more quick question. Is this the right IUPAC name?
2,4-dimethyl-2-pentene?
2,4-dimethyl-2-pentene?
- #6
ShawnD
Science Advisor
- 718
- 2
Looks right to me.
1. What is polymerization?
Polymerization is a chemical reaction in which small molecules, called monomers, join together to form long chains, called polymers. This process is key in the production of many common materials such as plastics, rubber, and fibers.
2. What are the different types of polymerization?
There are two main types of polymerization: addition polymerization and condensation polymerization. Addition polymerization involves the joining of monomers without the loss of any small molecules, while condensation polymerization involves the loss of small molecules, such as water, during the formation of the polymer chains.
3. What factors influence the rate of polymerization?
The rate of polymerization can be influenced by several factors, including temperature, concentration of monomers, and the presence of a catalyst. Higher temperatures and higher concentrations of monomers typically lead to faster polymerization, while the use of a catalyst can speed up the reaction even further.
4. What are some examples of polymers?
Some common examples of polymers include polyethylene, which is used to make plastic bags and bottles, polystyrene, which is used for packaging and insulation, and polyvinyl chloride, which is used for pipes and flooring. Other examples include natural polymers such as DNA, proteins, and rubber.
5. What are the potential applications of polymerization?
Polymerization has a wide range of applications, including the production of synthetic materials for everyday use, such as plastics, rubber, and fibers. It also has applications in the development of adhesives, coatings, and paints. Additionally, polymerization plays a crucial role in the production of pharmaceuticals, biodegradable materials, and advanced materials for use in industries such as aerospace and electronics.
Similar threads
- Replies
- 9
- Views
- 5K
-
Chemistry
- Replies
- 8
- Views
- 1K
-
Materials and Chemical Engineering
- Replies
- 1
- Views
- 3K
- Replies
- 3
- Views
- 1K
- Replies
- 16
- Views
- 1K
- Replies
- 1
- Views
- 2K
- Replies
- 3
- Views
- 3K
- Replies
- 8
- Views
- 4K
-
Biology and Chemistry Homework Help
- Replies
- 5
- Views
- 1K
-
Electromagnetism
- Replies
- 2
- Views
- 2K
Share: