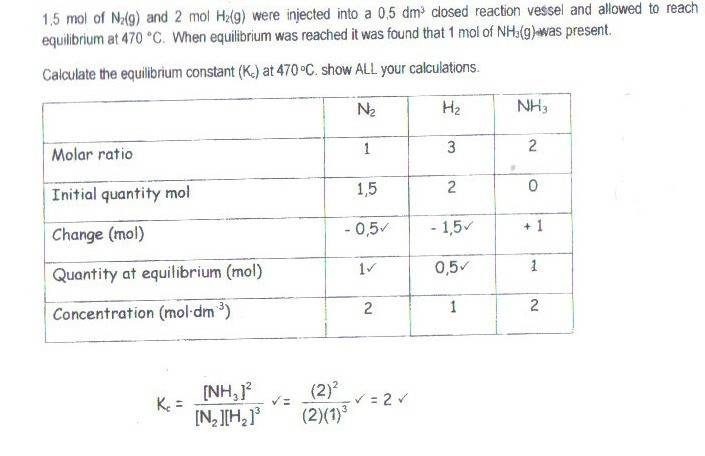
The discussion centers on understanding the molar ratios in a chemical reaction involving the production of ammonia (NH3) from nitrogen (N2) and hydrogen (H2). To produce 1 mole of NH3, 0.5 moles of N2 and 1.5 moles of H2 are required, based on the stoichiometry of the reaction. Participants clarify that the change in moles reflects the consumption of reactants and the production of products, with negative signs indicating the reactants are being used up. The concept of equilibrium is also touched upon, emphasizing that if NH3 decreases, it implies a reverse reaction where NH3 converts back into its reactants, necessitating an initial amount of NH3 for this process to occur. Overall, the conversation highlights the importance of understanding molar ratios and changes in chemical reactions.