Chemistry Definition and 993 Threads
-

Undergrad What is the composition of the Earth's exosphere?
Hello! My name is Fabrizio, I'm a high school student doing some research on the atmosphere. Right now, I'm trying to figure out how much matter escapes into space via Jean's Escape. I've found several formulas, all of which require me to know what the amount of particles per m3 of a certain...- Fabrizio Vassallo
- Thread
- Atmosphere Chemistry Composition Physics
- Replies: 5
- Forum: Mechanics
-
S
What Happens to Reagents When Sodium Hydride Is Added?
- sd34
- Thread
- Chemistry Reaction
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

2020 US National Chemistry Olympiad
Summary: Thread for discussing the USNCO and how to do well on it. More specifically, tips for doing well on the national round and making the study camp are welcome. Anyone with experience with the olympiad is welcome to post, especially previous study camp qualifiers and honors/high honors...- trienebutdiene
- Thread
- Chemistry Olympiad
- Replies: 1
- Forum: STEM Academic Advising
-
K
Decomposition reaction for theophylline, hexobarbital natrium
Hello! There is a problem to write chemical reactions that goes with substances if they are not stored properly. For example theophylline should be saved from light and though I am trying to find its’ reaction with hv(light) but failed. Please help with some good reference Many thanks in advance -

What Happens to Bromines in a Double Bond in Organic Chemistry?
I think that the double bond would be broken in some way but what happens to the two bromines? do they remain in the ring? (or does HBr forms? is it possible?)- KristinaMr
- Thread
- Chemistry Organic Organic chemistry
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

How to get the balanced redox equation in this sample problem?
Summary: Our chemistry teacher gave a sample problem regarding balancing a redox equation. I managed to proceed to a considerable distance, but I can't seem to understand how to get the final balanced equation. Please help me out. The problem is; reaction of K2Cr2O7 with SO2 in the presence of...- nineteen
- Thread
- Balancing equations Chemistry Homework Redox
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-
N
Do chemists think differently than physicists?
Summary: Do chemists think differently than physicists? And should I Change my subject in University... Hey guys, I'm asking myself this question in hoping to find myself in life… some advice and ideas would be really helpful. I'm from Europe and I'm actually studying chemistry. I still Need...- needsomeadvicemb
- Thread
- Chemistry Difference Physicists Physics Thinking
- Replies: 33
- Forum: STEM Academic Advising
-

Undergrad Helium Hydride data, Early universe chemistry evidence?
I don't think there is a thread on this? R Gusten et al, Nature, 2019, DOI: 10.1038/s41586-019-1090-x- pinball1970
- Thread
- Chemistry Data Early universe Evidence Helium Universe
- Replies: 6
- Forum: Cosmology
-
E
Entalphy of Formation: Why Use Water at Std Cond?
why do we use the entalphy of formation of water at standard conditions to calculate the entalphy of a reaction even if water is not gas at 1bar and 298K -

How to convert pressure (Bar) to flow rate (L/min)?
hii, I'm try to calculate the pressure (in bar) needed for a certain flow rate 3 L/min of Argon gas given the following setup: - I have a tank of Argon gas and the line pressure can be between 0 bar to 5 bar. - The gas will flow out to the open air in the end. - My pipe has an internal radius...- avishay
- Thread
- Chemistry Convert Flow Flow rate Gas Physics Pressure Rate
- Replies: 5
- Forum: Mechanical Engineering
-
A
Atomic High School Chemistry Textbook Recommendation
Can someone please tell me what high school chemistry textbook that studying about octet rule is best for an idiot like me?- askor
- Thread
- Chemistry High school Recommendation School Textbook
- Replies: 1
- Forum: Science and Math Textbooks
-
M
Chemistry Molar solubility lab questions
I’m confused on how to calculate molar solubility because I don’t see what’s the difference between that and Ksp.- Madelin Pierce
- Thread
- Chemistry Lab Solubility Thermodynamics
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

Programs Can I Use My Chemistry Background for Research in Physics?
I did not opt for physics for my degree course (BSc), I am sort of regretting it. Now, I'd rather want to get close to physics as much as possible. I like physical chemistry, I was thinking I should change my area of interest from neuroscience to physics cause honestly physics is much more...- DrTherapist
- Thread
- Chemistry Physics
- Replies: 22
- Forum: STEM Academic Advising
-
C
Does temperature increase when water is boiling at 100C and pressure is increasing......?
Does temperature increase when water is boiling at 100C in a closed system? I am picturing a scenario where I am boiling water in a pot to make pasta. However, I decide to close the pot as the water is still boiling. By doing this I am sealing away the system of study from the environment. Thus...- Carbon273
- Thread
- Boiling Chemistry Heat increase Physics Temperature Thermodyamics Water
- Replies: 8
- Forum: Mechanical Engineering
-
K
Why is DeltaG Negative for H2O(l) to H2O(g) at Equilibrium?
Calculate deltaG for the reaction: H2O(l) = H2O(g). 100 degrees celsius, water is clean. P(H2O) = 0.1 bar. Given that it is an equilibrium, I'd think that deltaG would be zero. But the answer is in fact negative. How is that possible?- Kqwert
- Thread
- Chemistry Energy Free energy Gibbs Gibbs free energy
- Replies: 8
- Forum: Biology and Chemistry Homework Help
-

Predictive Abilities of Chemistry
Hypothetically, if a chemist/physicist knew all the properties of hydrogen and all the properties of oxygen but knew nothing of water or its properties, would he be able to predict that combining 2 atoms of hydrogen and one atom of oxygen would produce a substance called water and its properties?- Jim Lundquist
- Thread
- Chemistry
- Replies: 18
- Forum: Chemistry
-

What tutorial for Resonance structures?
Hello everybody, I am stuck with drawing resonance structures for different types of compounds. Do you know any tutorials or any methods to master drawing resonance structures? If yes, please be kind enough to drop some links and statements below. Thank you very much in advance. -
O
Are physics and fundamental chemistry only necessary to become a physicist?
Does the understanding and application of physics and fundamental chemistry is only required to be physicist? Or you have to be be equally apt in chemistry??- Orion73
- Thread
- Chemistry Fundamental Physicist Physics
- Replies: 9
- Forum: STEM Academic Advising
-
A
Undergrad Can you change the state of matter by increasing the speed?
We all know that the state of matter can be changed by increasing temperature or by applying pressure. And, all of these, in some way lead to a change in the particles' speed. The speed of the particle decides the state of matter. So, if we were to throw an object at very high speeds, like 50%...- Archmundada
- Thread
- Change Chemistry Gases Increasing Matter Particle motion Solids Speed State State of matter
- Replies: 4
- Forum: Mechanics
-

Why do we have to memorize Lewis structures of ....
Why do we have to memorize the structures of all the nitrogen oxides? Isn't there any way to understand about the Lewis structures of all the nitrogen oxides and their weird ways of making bonds? -

Chemistry How can I find the molecular formula in this problem?
Homework Statement In an organic compound "A" only C,H and O is present. 1.22g of A is completely burned and it gives 0.84g of CO2 and 0.54g of H2O. If the relative molecular mass of "A" is 123. Find the molecular formula of A. [/B] I have showed my attempt at solving this problem, but I think...- nineteen
- Thread
- Chemistry Formula Molecular
- Replies: 10
- Forum: Biology and Chemistry Homework Help
-

Chemistry Finding the Chemical Formula and Relative Molecular Mass
My chemistry teacher gave me this problem. I tried and tried, but I couldn't figure it out and the deadline is tomorrow. Please help me out here friends. 1. This is the problem : In a compound which is made of element Y, weight percentage of Y is 72% and N( Nitrogen) is 28%. Also, 3 Y atoms...- nineteen
- Thread
- Chemical Chemical formula Chemistry Formula Homework Mass Molecular Relative
- Replies: 13
- Forum: Biology and Chemistry Homework Help
-

Why do we study or learn about ideal gases?
We are learning the lesson about gases/gaseous states at our school and I couldn't help but wonder, why learn about IDEAL GASES... How do ideal gases help us to analyze about real gases? -
M
Chemistry Lab Titration curve calculations
Homework Statement Homework EquationsThe Attempt at a Solution I don’t have a clue on how to do the calculations. I know the equivalency point is at the steepest part of the derivative graph. Please don’t say just figure it out, I truly need help. I haven’t learned titrations in lecture yet...- Madelin Pierce
- Thread
- Calculations Chemistry Curve Lab Titration
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
T
Does sodium bicarbonate solution release CO2 when heated?
Homework Statement Solid sodium bicarbonate decompose when it's heated above 50 celsius. However, when it is dissolved in water interactions of components changes. So what happens when sodium hydrogen carbonate solution is heated? Does it release carbon dioxide when temperature is above 50...- Tohtori huithapeli
- Thread
- Chemistry Co2 Heating Reaction Release Sodium Sodium bicarbonate
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
K
Understanding the Ionization of Salts
Hello, I am a bit confused re. solubility calculations. Calculate the solubility of Pb(OH)2 at pH 10. Setting up the expression for Ksp: Ksp = [Pb2+][OH-]2 = 8* 10^-17 (Ksp value from SI chemical data) pOH = 14 - 10 = 4, i.e. [OH-] = 10-4. 8*10-17 = [Pb2+][OH-]2. Solving for [Pb2+] we get... -
K
Chemistry Chemistry textbook for Physicists
Hello Being a professional physicist(Quantum field theory and HS theory) I'd like to learn chemistry for some reasons. I've already tried to find a nice Chemistry textbook but failed to find physicist friendly one. My last class on chemistry was in high school like 11 years ago already, so my...- Korybut
- Thread
- Chemistry Physicists Physics Textbook Textbook request Theoretical physics
- Replies: 3
- Forum: Science and Math Textbooks
-
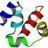
Researchers double the size of the DNA alphabet
Published this week in the journal Science, researchers report that they have devised a eight letter alphabet for DNA and RNA: The work builds off of previous work, which had expanded the genetic alphabet to six letters. The researchers call their eight-lettered nucleic acids "hachimoji,"...- Ygggdrasil
- Thread
- Biology Chemistry Dna Rna Synthetic biology
- Replies: 5
- Forum: Biology and Medical
-
D
Chemistry How do you calculate moles to neutralise oxalic acid?
Homework Statement calculate the number of moles of potassium manganate required to neutralise oxalic acid. 2KMn)4- + 5H2C2O42-+ 16H+ --> 2Mn2+ + 10CO2 + H2O Homework Equations n = c x V n= m/Ar The Attempt at a Solution Moles of oxalic acid = 5x10-4 Volumeol of KMnO4 = 0.01085dm-3...- Daniel2244
- Thread
- Acid Chemistry Moles Titration
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
M
Chemistry Equilibrium Partial Pressures
Homework Statement Kc = 4.15 x 10-2 at 356°C for PCl5(g) ↔ PCl3(g) + Cl2(g). A closed 2.00 L vessel initially contians 0.100 mol PCl5. Calculate the total pressure in the vessel (in atm to 2 decimal places) at 356°C when equilibrium is achieved. Homework Equations PV=nRT Kp= Kc(RT)^change in...- Madelin Pierce
- Thread
- Chemistry Equilibrium Partial Partial pressure
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
M
Kc = 1.88 for 2HI(g) ↔ H2(g) + I2(g)
Homework Statement Kc = 1.37 for HI(g) ↔ 1/2 H2(g) + 1/2 I2(g). What is Kc (to 3 decimal places) for H2(g) + I2(g) ↔ 2HI(g)? Homework Equations Kc= Products/Reactants, Ice Tables The Attempt at a Solution I tried an ice table, but I’m not sure what to do with the Kc value I’m given when I...- Madelin Pierce
- Thread
- Chemistry Equilibrium Equilibrium constant
- Replies: 4
- Forum: Biology and Chemistry Homework Help
-

Calculate the "surface energy" of succesively smaller cubes
Homework Statement NaCl has a density of 2.16 g/cm3 and a surface energy of 2x10-5 J/cm2. Calculate the surface energy of 1 g of NaCl. Initially the complete 1 g is in the form of a single cube (of mass 1 g) and progressively broken into smaller cubes with sides of 0.1 cm, 0.01 cm, 10 μm, 1 μm...- Jacob Daniel
- Thread
- Chem Chemistry Energy Surface energy Thermodynamics
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
M
Chemistry: dissolving aluminum metal in hydrochloric acid
Homework Statement Aluminum metal dissolves in hydrochloric acid. What volume, in mL, of 1.58 M HCl is needed to completely dissolve 3.200 g of aluminum?(Hint: write the single-replacement reaction first) Homework Equations V[1]M[1]=V[2]M[2] The Attempt at a Solution I got the...- Madelin Pierce
- Thread
- Acid Aluminum Chemistry
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
D
How do I calculate the enthelpy of formation using combusion data
The enthalpy of formation equation: ΔHƒ°reaction=∑Δhƒ°(products)-ΣΔHƒ°(reactants) When using the enthalpy of formation equation you get: -2010-((-394x3)+(-286x4))= 316Kjmol-1 However, this answer is wrong. On the mark scheme, the answer is -316Kjmol-1. So I used the enthalpy of combustion...- Daniel2244
- Thread
- Chemistry Data Enthalphy Formation
- Replies: 9
- Forum: Biology and Chemistry Homework Help
-

Why doesn't the amount of Cl increase?
Homework Statement Homework Equations Formula of Kp and Kc The Attempt at a Solution You will easily be able to identify that the reaction will proceed in the forward direction when some inert gas is introduced. So,my question is that why won't the amount of chlorine formed increase in...- navneet9431
- Thread
- Chemical equilibrium Chemistry Equilibirium increase
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-

Programs Working at the center for Computational Quantum Chemistry
Hello, I'd like to work in the above named center at the University of Georgia in Athens, Georgia under Dr. Henry Schafer. However, he's a professor in the chemistry department. I'm guessing if I go in the university as a physics graduate student I can't have him as a Ph.D advisor. However, is...- gimak
- Thread
- Center Chemistry Computational Quantum Quantum chemistry
- Replies: 12
- Forum: STEM Academic Advising
-

What is acet in organic chemistry?
What does it mean and why do acetophenone and acetic acid have it in their name ?- Logic hunter
- Thread
- Chemistry Organic Organic chemistry
- Replies: 3
- Forum: Chemistry
-

What are some good books on inorganic chemistry?
I'm a weak student in chemistry, by 'weak' I mean I lack some base I think. May please suggest some good books on inorganic chemistry? I want to study p-block, d- and f-block elements along with their important compounds and their preparation. Book should be concise as for me as a weak student...- Adesh
- Thread
- Books Chemistry Inorganic chemistry
- Replies: 3
- Forum: Science and Math Textbooks
-
Programs Should a physics major take general chemistry?
I’ve already taken intro chemistry with a lab, and general chemistry 1. Unlike most universities, my college doesn’t require chemistry for physics majors. Should I take general chemistry 2? Would it really benefit me, or should I spend my time elsewhere? Edit: I’m referring to the standard...- astroman707
- Thread
- Chemistry General General chemistry Major Physics Physics major
- Replies: 10
- Forum: STEM Academic Advising
-

Make a Thermos: Calculate Final Water Temperature
Homework Statement Hello, As part of my engineering orientation class, my team and I have to make a device that can keep a beaker of water warm. We have a list of materials we can and cannot use, but my team and I have decided on aluminum foil and maybe wood. We don't actually have to build...- Martinez43
- Thread
- chemistry phyiscs thermal conductivity
- Replies: 4
- Forum: Engineering and Comp Sci Homework Help
-

The relationship between pH and pOH at 70 ◦C/ 343.15 K
Homework Statement The relationship between pH and pOH, for pure water, at 25 ◦C is 14 = pH + pOH What is the corresponding relationship between pH and pOH, for pure water, at 70 ◦C? Homework Equations Le Châtelier's principle? [H+][OH-] = 10-14 [10-7][10-7] = 10-14 (25 ◦C) [10-n][10-m] =...- ChristinaMaria
- Thread
- Chemistry Ph Relationship
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-

Which one is more reactive, CO2 or NO2?
Homework Statement Which one is more reactive, CO2 or NO2? Homework Equations The Attempt at a Solution I drew the Lewis structures of these species and found there is one delocalized electron in NO2, so I think NO2 is more reactive. Am I correct?- christang_1023
- Thread
- Chemistry Co2 Reaction
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
J
Al has higher 2nd ionisation energy — why?
Homework Statement The question's in the screenshot attached. Homework EquationsThe Attempt at a Solution I don't know why (B) would be right. I feel like since for Mg's second ionisation energy, it is going from Na to Ne, and Ne is another energy level, Mg's 2nd ionisation energy should be...- JessicaHelena
- Thread
- Ap chemistry Chemistry Energy Ionisation energy
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
L
Calculating pH with and without ionic strength
Homework Statement There are four things to be calculated: -pH of 0.00196M solution of HCl in water, without taking ionic strength into account, -The same thing but taking ionic strength into account -pH of 0.00196M solution of HCl in Na2SO4, without taking ionic strength into account, -And...- Labchem3
- Thread
- Chemistry Ionic Ph Strength
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-
A
Which is more stable: cyclobutane or cycloheptane?
Homework Statement [/B] Which is more stable cyclobutane or cycloheptane? Homework Equations [/B] [(n-2)180)]/n = angle The Attempt at a Solution [/B] So the closer the angle is to 109.5 the more stable (except for cyclopentane vs cyclohexane). n = number of carbons [(7-2)180)]/7 = 128.57...- AMan24
- Thread
- Chemistry Stable
- Replies: 1
- Forum: Biology and Chemistry Homework Help
-

How to like chemstry? [Looking for inspiration]
Dear friends, I've been having trouble focusing on chemistry with all these boring teachers I get at school. I want some help to increase my interest for chemistry so that I can research about chemistry alone at home. Dear friends, what are the ways I can think of chemistry and how should I...- nineteen
- Thread
- Chemistry
- Replies: 10
- Forum: STEM Academic Advising
-
N
Is Melted Bismuth safe to wear as a necklace?
Is melted bismuth safe to wear pretty much 24/7 around your neck ? I don't want to get a super low radiation hyper bug cancer fungus to appear after 30 yrs... (It touches your skin permanently unless you're running ofc) On top of that, can I smelt bare bismuth (99%) in my kitchen or should I...- Natrium420
- Thread
- bismuth chemistry health radiation
- Replies: 19
- Forum: Chemistry
-
M
How are the isomers of tris(acetylacetonato) chromium(III)?
Homework Statement How to draw it? Can someone tell me the electronic configuration of the chromium atom in tris( acetylaceato)chromium( III) please Homework Equations N/A The Attempt at a Solution N/A- mimi88
- Thread
- Chemical Chemistry Isomers
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
J
Chemistry lab - limiting reagent - Can someone verify?
Homework Statement Suppose that, for reaction 4, you could not find the bottle of 7 M H2SO4 so you added 5.00 mL of the 1.00 M H2SO4 instead. How would this impact your final yield of Copper. (Show with calculations how this would impact the limiting reagent.) Homework Equations CuO(s) +...- John Ker
- Thread
- Chemistry Lab
- Replies: 3
- Forum: Biology and Chemistry Homework Help
-
L
Electrolysis of NaHCO3 solution with lead electrodes
Hey. If i carry out electrolysis of saturated sodium bicarbonate solution with lead electrodes, will the lead electrodes will dissolve to form lead carbonate (or basic lead carbonate if temperature is higher) or the lead ions will migrate from anode to cathode and deposite there? I think that...- LeoPhoeniX
- Thread
- Chemistry Electrochemistry Electrodes Electrolysis Lead Physics
- Replies: 2
- Forum: Chemistry