Entropy Definition and 999 Threads
-

Graduate Significance for LQG of Sen's result on entropy of black holes?
Sen 2013 says, How serious a problem is this for LQG? Does this mean that LQG doesn't have GR as its semiclassical limit? Does that mean it's a dead theory, or maybe just that it needs to be modified? Is the technique using Euclidean gravity reliable? Since I'm not a specialist, I'd be...- bcrowell
- Thread
- Black holes Entropy Holes Lqg Significance
- Replies: 8
- Forum: Beyond the Standard Models
-
F
Why is Cpln(T2/T1) Zero for Isothermal Entropy Change?
Can someone explain to me why Cpln(T2/T1) = 0? Thank you.- freshbox
- Thread
- Change Entropy Isothermal
- Replies: 2
- Forum: Introductory Physics Homework Help
-
D
Undergrad How Do You Calculate the Change in Entropy When Ice Melts in Water?
In 250gr water at 23 oC(degrees celsius) we throw in 27gr ice at 0 oC. Find the change in entropy. (sorry if that was a bad translation but English is not my native language) The answer is 0.78 calories/oC. But I'm not sure how do this. The formula I have for entropy is: S=δQ/T I find the...- devil0150
- Thread
- Change Entropy
- Replies: 1
- Forum: Thermodynamics
-
E
Graduate Entropy increase,coarse-grained vs. Landauer's principle justification
First off, just clarify that I have a very, very superficial knowledge of Physics, so my apologies if my question is based on an obvious misunderstanding of the basic principles underlying the second law of thermodynamics or if it has a rather simple answer. The doubt that I have is related...- Evaristo
- Thread
- Entropy Principle
- Replies: 3
- Forum: Thermodynamics
-

Graduate Loop Quantum Gravity and entropy
How specifically does LQG explain how the universe can oscillate from big bang to big crunch ad infinitum? Wouldn't the total energy able to be used as work decrease after a couple of bounces? Am I simply misunderstanding or making a false assumption about what LQG's premises are? I've not...- Digitalism
- Thread
- Entropy Gravity Loop Loop quantum gravity Quantum Quantum gravity
- Replies: 8
- Forum: Beyond the Standard Models
-
L
How Is Entropy Calculated for a Density Matrix with Eigenvalues 0 and 1?
Homework Statement Calculate entropy for density matrix with eigenvalues ##0## and ##1##. Homework Equations ##S=-\lambda_1 \ln \lambda_1-\lambda_2 \ln \lambda_2## where ##\lambda_1## and ##\lambda_2## are eigenvalues of density matrix. The Attempt at a Solution How to calculate...- LagrangeEuler
- Thread
- Entropy Quantum Statistic
- Replies: 8
- Forum: Advanced Physics Homework Help
-
V
Graduate Relating physics forces and entropy
Consider proton and electron are separated in space at some finite distance.Now suppose they released then the decrease in potential energy equals kinetic energy but as electrons or protons are accelerated they emit em and lose some gained kinetic energy.Also the mass is another form of...- vishvajeet
- Thread
- Entropy Forces Physics
- Replies: 2
- Forum: Thermodynamics
-
T
Graduate Entropy and electromagnetic radiation
I don't understand this: According to what modern physicists believe to be true, there is entropy that slowly converts all energy of the universe into heat that cannot do any work. Than this heat is radiated as infrared light into space. Correct? Besides infrared heat radiation, start also...- tarakan
- Thread
- Electromagnetic Electromagnetic radiation Entropy Radiation
- Replies: 3
- Forum: Electromagnetism
-
S
Entropy of a rubber-band (force determined by entropy or energy)?
Homework Statement The resultant of a experimental run of a force (N) vs temperature (K) gives me a slope of 0.0039x - 1.0929. The slope has a (∂f/∂T)l = (∂S/∂l)T Homework Equations Equation (11) attached as PNG file We can obtain all the quantities of the right side of Eq. (11)...- sdnnet3
- Thread
- Energy Entropy
- Replies: 2
- Forum: Advanced Physics Homework Help
-
S
Graduate How meaurements increase entropy?
¿ How meaurements increase entropy? The decoherence before the measurement increase entropy but collapse returns the state of the system to a pure state. ¿Why then measurements increase entropy- StarsRuler
- Thread
- Entropy increase
- Replies: 9
- Forum: Quantum Physics
-
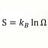
Entropy Of Mixing and Specific Heat
Homework Statement 100g of lead, specific heat .0345 cal/g/C at 100 C is mixed with 200 g of water at 20 C. Find the difference in entropy of system at end from value before mixing. Note: I think the book has wrong answer of 1.42 cal/K Homework Equations Before mixing entropy for water =...- morrobay
- Thread
- Entropy Heat Mixing Specific Specific heat
- Replies: 17
- Forum: Introductory Physics Homework Help
-
G
Graduate Will the fusion reactor ITER decrease entropy?
ITER is the fusion reactor in southern France that hopefully will come online in 2018. According to wiki http://en.wikipedia.org/wiki/ITER The ITER fusion reactor itself has been designed to produce 500 megawatts of output power for 50 megawatts of input power. Does this mean that if...- g.lemaitre
- Thread
- decrease Entropy Fusion Fusion reactor Iter Reactor
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
B
Graduate Which has least entropy- living organism or same mass as chrystal?
Which has the least entropy? A 1kg diamond vs 1kg living organism I've gather that living things are generally at a state of low entropy compared to the same matter in other configurations- as though complexity goes hand in hand with low entropy. Yet a perfect chrystal is supposedly low...- bcrelling
- Thread
- Entropy Mass Organism
- Replies: 11
- Forum: Thermodynamics
-
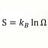
Entropy For Irrevesible Process
Homework Statement Two 100g Aluminum cubes with T1 = 80° C and T2 = 20°C Specific heat of Al is .22 cal/g/°C. The cubes are placed in contact and in short period of time a quantity of heat, dQ , is transferred from T1 cube to T2 cube. The cubes temperatures now are 75°C and 25°C. After a...- morrobay
- Thread
- Entropy Process
- Replies: 9
- Forum: Introductory Physics Homework Help
-
G
Entropy problem involving saltwater uptake into a cell
Homework Statement For public health reasons, you are investigating small systems that turn sea water into drinking water. One portable system takes a volume of saltwater and produces two thirds that volume of freshwater with an increased concentration of salt in the other third of the volume...- GoGoGadget
- Thread
- Cell Entropy
- Replies: 5
- Forum: Introductory Physics Homework Help
-
A
Undergrad Why is entropy a driving force for a reaction
I understand the Gibbs Free Energy equation that was drilled into us in Chemistry class but not even my teacher could explain what entropy had to do with free energy, and furthermore, why entropy is a driving force for a reaction. From what I understand, entropy is the distribution of energy...- arp001
- Thread
- Entropy Force Reaction
- Replies: 11
- Forum: Thermodynamics
-
B
Graduate Move A->B What about entropy change?
move A-->B ...What about entropy change? Hi, I have a physical question regarding entropy, temperature, internal energy and mechanical energy. The situation is following: Two objects are located inside a one dimensional frictionless and adiabatic space where object “a” is an observer that...- bolly
- Thread
- Change Entropy
- Replies: 25
- Forum: Thermodynamics
-
D
Graduate Can a Black Hole's High Entropy State Coexist with Supercondensed Matter?
How can a black hole have a high entropy state if the matter inside it is in a supercondensed form with maximum density,which means less degrees of freedom therefore less entropy than normal matter.Like in neutron stars we have low entropy with the neutron degenerate matter and yet a black hole...- Double-Slit
- Thread
- Black hole Entropy Hole
- Replies: 14
- Forum: Cosmology
-
K
Graduate Entropy change during wet compression
I have a quick question. Consider the process S2 to S1 in the figure below Since this represents wet (isentropic) compression, mathematically we have ΔS=0 (assuming adiabatic compression). But if we consider the process in a physical way, we are going from a region of less disorder...- kulkajinkya
- Thread
- Change Compression Entropy
- Replies: 6
- Forum: Thermodynamics
-
K
Graduate What is the Shannon Entropy of the String 'QXZ'?
Shannon entropy of "QXZ" Hello everyone. I am trying to determine the Shannon entropy of the string of letters QXZ, taking into consideration those letters' frequency in English. I am using the formula: [SIZE="4"]H(P) = –Ʃ pilog2pi What's puzzling me is that I am expecting to calculate a...- Karl Coryat
- Thread
- Entropy Shannon entropy
- Replies: 2
- Forum: Set Theory, Logic, Probability, Statistics
-
B
Graduate Does entropy drive our conscious experience of time?
From what I can gather, the laws of physics are time sysmmetrical- the exception being the second law of thermodynamics. So could it be that our whole experience of time is driven souly by increasing entropy? Assuming that we live in a deterministic universe(controversial I know) all past...- bcrelling
- Thread
- Drive Entropy Experience Time
- Replies: 5
- Forum: Thermodynamics
-
H
Graduate What is the Relationship Between Entropy and Order in Physical Systems?
In my TD classes I have to deal with entropy. But what does it really mean? I know that it is the degree of disorder and the equation S=q/t. But what is this disorder? How can a physical system be aware of order and disorder since it is an abstract concept?They say that solids have high order...- HARI A
- Thread
- Disorder Entropy Physical Systems
- Replies: 8
- Forum: Thermodynamics
-
C
Is the Universe Organized or Chaotic in its Younger Years?
I was thinking maybe the universe isn't in the chaotic state its meant be in. Rather a more organised state and the universe is still in it's younger years. This is just a thought any insight would be great.- capcom1983
- Thread
- Entropy Universe
- Replies: 11
- Forum: Cosmology
-
D
Entropy change when converting water to steam
Homework Statement At constant atmospheric pressure, 20g of water at 30C is converted into steam at 250C. Assume the heat capacity of liquid water is constant at 4.2 J/gK and the heat of vaporization at 100C is 2260 J/g. The molar heat capacity of water vapor at constant pressure is given by...- doombanana
- Thread
- Change Entropy Steam Water
- Replies: 1
- Forum: Introductory Physics Homework Help
-
W
Entropy change in isochoric process
Homework Statement Hi everyone In an isochoric process ( a solid heat body with T1 temp. connected with heat bath with T2 temp) we could calculate its entropy change(heat body) from dS = Cv/T dT and entropy change of heat bath from dS = dQ / T2, using dQ = Cv dT in 1st equation ( i...- wowowo2006
- Thread
- Change Entropy Isochoric Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
B
Entropy Change of a Monatomic Ideal Gas Compressed Adiabatically
Homework Statement A monatomic ideal gas is compressed adiabatically. What is ΔS of the gas? n = 1.00 vi = 10.00 L vf = 4.00 L Constant External Pressure = 50.00 atm Homework Equations ΔU = q + w = 0 + (-PextΔV) ΔS = ∫[(qrev)/T]dQ The Attempt at a Solution In order to...- Basis
- Thread
- Change Compressed Entropy Gas Ideal gas
- Replies: 5
- Forum: Introductory Physics Homework Help
-

The Entropy of Information Source
In the paper "A Mathematical theory of Communication" by Shannon I couldn't understand a theorem (Theorem 4) under the topic "The Entropy of Information Source"- Here 'H' is the entropy. Can you explain me how we get this theorem? What is this n(q)? I couldn't understand the sentence...- iVenky
- Thread
- Entropy Information Source
- Replies: 4
- Forum: Electrical Engineering
-
A
Graduate Boltzmann's entropy/ Microcanonical Entropy
Hey guys, i am reading something about entropy. :confused: And got a question. The Boltzmann entropy is defined by: S=k\cdot \ln{W} W is the number of microstates connected to an given macrostate. The entropy of the microcanonical ensemble(fixed given Energy) is defined by...- Abigale
- Thread
- Entropy
- Replies: 1
- Forum: Thermodynamics
-
W
Entropy Generation of an Electric Motor: Analyzing du/dt & dS/dt
For the following question, the solution manual sets du/dt (change of internal energy) = 0. Moreover, it also set the change of total entropy to zero. Why is that? An electric motor under steady load draws 9.7 amperes at 110 volts; it delivers 0.93 kW of mechanical energy. The temperature of...- wynnl
- Thread
- Electric Electric motor Entropy Generation Motor
- Replies: 1
- Forum: Introductory Physics Homework Help
-

Graduate A Gravitational Entropy proposal from Ellis, Tavakol, and Clifton
It's interesting that the entropy of the gravitational field (i.e. the entropy of geometry) has never been satisfactorily defined. Since geometry is such an important part of the picture near the start of expansion, this means that total entropy around then has been undefinable, leaving... -
G
Entropy Properties: Correct & Incorrect Expressions
hello, i have been given two formulae for the entropy of an ideal gas undergoing a reversible process. the correct expression is: S = Nk(s0-ln({\frac{N_0}{N}})^{5/2} + ln({\frac{T}{T_0}})^{3/2} + ln({\frac{V}{V_0}})) where s_0 is a constant, N the number of particles, T the...- Guffie
- Thread
- Entropy Properties
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Entropy Variation - System, Neighborhood and Universe
I learned ΔSuniv = ΔSsys + ΔSneib If ΔSuniv > 0 the process is spontaneous. But ΔS = Q/T right? Shouldn't Qsys = -Qneib? (The heat released by the system = - heat released by the neighborhood) I cannot understand why this is wrong! If it were the case, ΔSuniv would be always zero... -
C
Graduate The photon, time and entropy
The photon, "time" and entropy (ignore original title, in fact id appreciate if a mod would change it. It was not my intention to have a conversation about time, lol!) Can we view a closed system of just photons, as ever, or usually, being subject to entropy (defined by the whole 2nd law...- Curious45
- Thread
- Entropy Photon Time
- Replies: 16
- Forum: Quantum Physics
-
S
Graduate Is There a Relationship Between Phase Space and Entropy in Particle Decay?
Is there any relationship between phase space and entropy? regarding to decay of particles- shakeel
- Thread
- Entropy Phase Phase space Space
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
T
What is the role of Shot and Scold in the entropy equation?
Homework Statement In the entropy equation 1S2 + ∫δQ/T = S2 - S1, what is the difference between 1S2 and ∫δQ/T? So for example if we have two reservoirs, one hot and one cold and heat is transferred in between we have: Shot = Q/Thot Scold = Q/Tcold Where do the Shot and Scold terms...- theBEAST
- Thread
- Confused Entropy
- Replies: 4
- Forum: Introductory Physics Homework Help
-
C
Graduate Poincaré recurrence and maximum entropy
Fluctuation theorem says that there will be fluctuations in microscopic scale that results local decreases in entropy even in isolated systems. According to the Poincaré recurrence theorem, after sufficiently long time, any finite system can turn into a state which is very close to its initial... -
C
Blackbody Radiation - Entropy and Internal Energy
Homework Statement Expression for the entropy and internal energy of black body radiation. Using the below relations: Homework Equations Total free energy for black body: $$ F = (k_b TV/\pi^2) \int k^2 ln[1-exp(-\hbar ck/k_b T)]dk $$ Relationship between partition function and internal...- chris_avfc
- Thread
- Blackbody Blackbody radiation Energy Entropy Internal Internal energy Radiation
- Replies: 4
- Forum: Advanced Physics Homework Help
-
P
Graduate Negative temperature and entropy
we know the entropy in each process increases is it true for absolute negative temperature?- persia7
- Thread
- Entropy Negative Temperature
- Replies: 8
- Forum: Thermodynamics
-
D
Graduate Entropy of a continuous system
How could the entropy of a continuous system, like the electromagnetic field, be defined? Obviously you can't use something like the log of the phase space volume, but I can't think of anything that would work.- dEdt
- Thread
- Continuous Entropy System
- Replies: 6
- Forum: Thermodynamics
-
T
Undergrad Why is entropy defined as a state property?
Hi,I m just beginner in physics,but actually I don need exact expression of physical laws in math language.I know,math is language of physics but still... So,I m looking for answer to my questions about origin of Clausius s entropy. I this article: http://www.panspermia.org/seconlaw.htm is...- thedy
- Thread
- Clausius Entropy
- Replies: 1
- Forum: Thermodynamics
-
P
How Do You Calculate Entropy Change in Thermodynamics Problems?
Homework Statement http://postimage.org/image/9artvc1h5/ see attached Homework Equations ΔS=Q/T The Attempt at a Solution The real issue I'm having is how to go about solving this, I think I'm going to need to use kinematics to find the time the gold is exposed to the air...- physninj
- Thread
- Entropy Thermodynamics
- Replies: 6
- Forum: Introductory Physics Homework Help
-
C
Graduate Big Bang & Entropy: Proof & Theories Explained
I am reading Cycles of Time by Penrose, page 71-72. Could someone explain if the universe started out with at a state of extraordinarily tiny entropy or a state of maximum entropy and what proof or theories do we have to justify this choice. I find the explanation in the book confusing... -
C
Graduate Entropy and Internal Energy in Fermi-Dirac statistics
What are the formulas for entropy and internal energy in Fermi-Dirac statistics, and how do I derive them?- cryptist
- Thread
- Energy Entropy Fermi-dirac Fermi-dirac statistics Internal Internal energy Statistics
- Replies: 2
- Forum: Thermodynamics
-
E
Entropy and Black Hole Temperature
Homework Statement In a previous problem I had to find the entropy of a black hole where I ended with this: S_{BH}=\frac{8 \pi^2 G M^2 k}{h c} Now I am to find the temp, given the energy of a black hole is mc2. Homework Equations T=(\frac{\partial S}{\partial u})^{-1} The...- erok81
- Thread
- Black hole Entropy Hole Temperature
- Replies: 1
- Forum: Introductory Physics Homework Help
-
R
Calc E as Fn of T for Ideal Paramagnet
Homework Statement The entropy of an ideal paramagnet is given by S=S_{0}+CE^{2}, where E is the energy (which can be positive or negative) and C is a positive constant. Determine the equation for E as a function of T and sketch your result. Homework Equations [tex] \frac{1}{T}=\frac{\delta...- relativespeak
- Thread
- Entropy Function
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Graduate Wave Function Collapse and Entropy
I don't want to argue about whether the notion of "wave function collapse" is a good way of understanding quantum mechanics, or not. For the purposes of this discussion, let's just adopt uncritically the naive approach to quantum mechanics, that: Between measurements, the system evolves...- stevendaryl
- Thread
- Collapse Entropy Function Wave Wave function Wave function collapse
- Replies: 49
- Forum: Quantum Physics
-
C
Undergrad Is entropy the same as heat capacity?
A formula for entropy: dS=dQ/dT This is a formula that represents the rate of change of heat with respect to temperature. Q=mcdT This is another formula that states how the temperature changes as heat is added. from this can we say that entropy is just specific heat capacity * Mass...- CraigH
- Thread
- Capacity Entropy Heat Heat capacity
- Replies: 6
- Forum: Thermodynamics
-

Graduate Understanding Expansion, Compression and Entropy Coefficients
So, until now I know: (DV/DS)p=(DT/Dp)s=a*T/cp*(rho) (enthalpy) (Dp/DT)v=(DS/DV)t=-a/k (helmoltz) (DS/Dp)t=-(DV/DT)p=-Va (gibbs) a=expansion coefficient k=isothermal compression coefficient cp=heat capacity at constante pressure I want to deduce DT/DV at constant entropy=(DT/DV)s...- tsuwal
- Thread
- Coefficients Compression Entropy Expansion
- Replies: 2
- Forum: Electromagnetism
-
D
Graduate Physics: Reconciling Entropy & Energy
I'm having a hard time reconciling two ideas in physics. One is that systems tend towards the maximum amount of disorder, or "entropy always increases". And, when two systems are brought together they are likely to be found at the energy E* which maximizes the number of states of the...- dm4b
- Thread
- Energy Entropy
- Replies: 17
- Forum: Thermodynamics
-
W
Graduate Why is Entropy a concave function of internal energy?
Hello I may well be all wrong about this but I am trying to understand from a microscopic point of view why Entropy is a concave function of internal energy. I found this in the following .pdf: http://physics.technion.ac.il/ckfinder/userfiles/files/avron/thermodynamics_potentials.pdf I...- Wentu
- Thread
- Concave Energy Entropy Function Internal Internal energy
- Replies: 4
- Forum: Thermodynamics