Hydrogen Definition and 1000 Threads
-
C
Is the Spontaneous Reaction of Hydrogen and Oxygen Dependent on Temperature?
Hydrogen and Oxygen "burn" Does anyone know the temperature at which this reaction becomes spontaneous? 2H2O--> 2H2 + O2 I can't find my book with thermodynamic data, but it should be cake for you guys. Thanks. -
S
Hydrogen Propellant: Can it Power an Engine?
Can hydrogen be burned and used to drive an engine?- sameev29
- Thread
- Hydrogen Propellant
- Replies: 2
- Forum: Materials and Chemical Engineering
-
A
Dynamics of electron in Bohr Hydrogen atom
1. Consider Bohr Hydrogen atom with counter-clockwise electron orbit in the xy plane with intial position r(0)=-a0y. The angular frequency of the orbit is w. Derive an expression for the position of electron at a later time t, r(t) in terms of a0 , w, t, x, and y. Homework Equations...- ani4physics
- Thread
- Atom Bohr Dynamics Electron Hydrogen Hydrogen atom
- Replies: 7
- Forum: Advanced Physics Homework Help
-
C
Understanding Probability Densities for Hydrogen Wave Functions
Homework Statement The problem, along with a solution, is attached as an image file. Homework Equations The Attempt at a Solution I have done the problem which was very straight forward. One simply had to look up the Rn,l and then plug in the appropriate quantum numbers. Since for...- CanIExplore
- Thread
- Functions Hydrogen Wave Wave functions
- Replies: 2
- Forum: Advanced Physics Homework Help
-
A
Graduate Real Hydrogen Atom: Exploring Electron Energy Effects
I've heard that the hydrogen atom that we originally learn about in QM that deals with the Coulomb force is an incomplete description. I'm having trouble understanding all of these effects. When describing the electron energy of the real Hydrogen atom, how do things like the zeeman effect...- Animak
- Thread
- Atom Hydrogen Hydrogen atom
- Replies: 5
- Forum: Quantum Physics
-
M
Undergrad Relation between kinetic energy and temperature for hydrogen
Hello I have a question about the relationship between kinetic energy and temperature for the hydrogen. In my professor's note, there is written that: (1/2)m*(v^2) = (1/2)*K*T where m is the mass, K the costant and T the temperature in Kelvin. My doubt is in the fact that on...- magodiafano
- Thread
- Energy Hydrogen Kinetic Kinetic energy Relation Temperature
- Replies: 2
- Forum: Thermodynamics
-
C
Graduate Hydrogen atom with discrete nonlinear Schrödinger equation
Hi everyone, How can I solve hydrogen atom with discrete nonlinear schrödinger equation? Could you help me with the mathematics of that, please?- cryptist
- Thread
- Atom Discrete Hydrogen Hydrogen atom Nonlinear Schrödinger Schrodinger equation
- Replies: 8
- Forum: Quantum Physics
-
D
Graduate Why is there still mostly hydrogen in pop I and II stars?
I've heard in the past something along the lines of "every atom in your body came from an exploding supernova". Yet, I can't see how that could be true when there is still such an abundance of hydrogen. Wouldn't any hydrogen in a star prevent it from reaching supernova stage? Assuming...- DaleSwanson
- Thread
- Hydrogen Stars
- Replies: 3
- Forum: Cosmology
-
X
Balmer series for atomic hydrogen lie
Homework Statement The first few lines of the Balmer series for atomic hydrogen lie at the wavelengths λ = 656.46, 486.27, 434.17, 410.29nm, ... Find a value for RH, the Rydberg constant for hydrogen. The ionization energy I is the minimum energy required to remove the electron. Find it from...- xregina12
- Thread
- Atomic Atomic hydrogen Hydrogen Series
- Replies: 1
- Forum: Introductory Physics Homework Help
-
S
Calculate Volume of Hydrogen Needed to Hydrogenate 50g Trans-Carveol
Homework Statement Calculate the volume required to hydrogenate 50g of trans-carveol at STP. Homework Equations PV=nRT m=152g The Attempt at a Solution I think I might have this one but just for clarification if I'm missing something. The amount of moles is 0.329 using the amount...- schoolboy10
- Thread
- Hydrogen Volume
- Replies: 5
- Forum: Biology and Chemistry Homework Help
-
D
<r> and <V(r)> in the Hydrogen Atom.
Hi. I'm a 3rd year undergraduate studying Applied Physics and I'm having some trouble with a problem concerning the Hydrogen Atom. This is my first post so please forgive the sloppy equations. Not really used to writing this stuff out without an equation editor handy! Anyway, the...- dirtyaldante
- Thread
- Atom Hydrogen Hydrogen atom
- Replies: 14
- Forum: Advanced Physics Homework Help
-
T
Graduate Intergalactic space travel and hydrogen blasting
Intergalactic space travel and hydrogen "blasting" To travel by spaceship to Andromeda (2.5 million light years) within a life time (say 50 years) you would have to travel with a much greater Lorentz factor than LHC protons, 50000 versus 7500. The LHC supposedly can melt a metric ton of copper...- teve
- Thread
- Hydrogen Space Space travel Travel
- Replies: 8
- Forum: Special and General Relativity
-
N
Undergrad Radiation that interacts with hydrogen
Hi there, Is hydrogen gas transparent to most radiation or is there a wavelength that interacts with hydrogen? I'm asking because I want to know if it's possible to "push" hydrogen in a vacuum with some kind of photons.- Northprairieman
- Thread
- Hydrogen Radiation
- Replies: 3
- Forum: Thermodynamics
-
L
Graduate Tokamak using elctrons instead of hydrogen nuclei
Q.) What would happen if a *reverse-Tokamak* was created, thus reversing the electromagnets to a negative charge and repelling electrons into each other instead of fusing hydrogen nuclei, what approximate temperature would need to be used and would it work? If so what would occur?- lewis1440
- Thread
- Hydrogen Nuclei Tokamak
- Replies: 1
- Forum: High Energy, Nuclear, Particle Physics
-
H
Undergrad The 6 hydrogen spectral line series'
I noticed that the hydrogen spectral lines are grouped into 6 series and given a value for n. I also noticed that each series was named after its discoverer but "coincidentally?" falls into a specific region of the EM spectrum so the Lyman series (n=1) of lines are all in the UV region, the...- Horseb0x
- Thread
- Hydrogen Line Series
- Replies: 4
- Forum: Quantum Physics
-
G
What is the mean speed of hydrogen atoms at 50K?
Homework Statement Dear all, I have to calculate the mean speed of hydrogen atoms, at temperature of 50K. Homework Equations Of course: <v>=\sqrt{\frac{8k_BT}{\pi m}} The Attempt at a Solution However, when I attempt to calculate it, m=\frac{2}{6.022x10^{23}} k_B=1.3807x10^{-23}...- Grand
- Thread
- Atoms Hydrogen Mean Speed
- Replies: 9
- Forum: Introductory Physics Homework Help
-
P
Unraveling Weak Field Stark Effect in Hydrogen
Homework Statement Considering the n = 2 states of hydrogen: In the absense of an external field, the four j = 1/2 states are degenerate. Using degenerate pertubation theory, I am supposed to show that for a very weak field the Stark effect shifts the energy levels by \mp \sqrt{3}a_0 e...- PHamnett
- Thread
- Field Hydrogen Stark effect Weak
- Replies: 1
- Forum: Advanced Physics Homework Help
-
F
Graduate What does it take for hydrogen to form from protons and electrons?
Hi What does it take for hydrogen to form from protons and electrons? I have searched quite a bit and the only information that I have so far is from this webpage http://www.Newton.dep.anl.gov/askasci/phy00/phy00843.htm from the webpage ------------------------------ To form a hydrogen...- fizz_it
- Thread
- Electrons Form Hydrogen Protons
- Replies: 2
- Forum: Electromagnetism
-
A
Why Are Absorption Lines Observed Only in the Lyman Series for Hydrogen?
When white radiation is passed through a sample of hydrogen gas(atoms assumed actually) why are absorption lines observed in Lyman series only? The corresponding photon energy range for visible light(380-780nm) are 1.59-3.27eV which should cause transitions of electrons in the first excited...- aim1732
- Thread
- Absorption Absorption spectrum Hydrogen Spectrum
- Replies: 4
- Forum: Advanced Physics Homework Help
-
X
Comparing Power Generation Potentials of Hydrogen and Xenon
Generally the working fluids in a power generating system is either water (steam) or air. This I imagine is because they are widely available fluids. The specific heats of these ranging from some 1 - 2 kJ/kg-K. For the sake of argument, let's assume that hydrogen or xenon are equally...- XIX
- Thread
- Generation Hydrogen Potentials Power Power generation Xenon
- Replies: 4
- Forum: Mechanical Engineering
-
D
Graduate Average Total Energies of Isolated Hydrogen Atom's Electron & Proton
I wanted to know the average total energies of an isolated hydrogen atom's electron and proton separately. I came across a lot of equations I could try to use, but I figured I'd ask to see if this information has already been approximated. Thanks- dirtyd33
- Thread
- Average Electron Energies Hydrogen Proton
- Replies: 2
- Forum: Atomic and Condensed Matter
-
A
Graduate Hydrogen atom (HELP ME FAST PLEASE)
Hi, I'm trying to figure out the solution for the ground state of the hydrogen-atom, however it is not going well. As far as i know, you can supress the angular dependence, because the states of hydrogen (or at least some of them) are spherically harmonic. This way the schr. equation just...- aaaa202
- Thread
- Atom Hydrogen Hydrogen atom
- Replies: 4
- Forum: Quantum Physics
-
B
Time evolution of hydrogen wave function that is not an energy eigenfunction
I recently had a probelm in QM to find the time evolution of a hydrogen prepared in a state with a wave function that is not an energy eigenfunction: specifically, psi = Y21*R2p where Y is then the D spherical harmonic. Of course, n=2 hydrogen doesn't have d oribtals. So the problem is I...- bier0134
- Thread
- Eigenfunction Energy Evolution Function Hydrogen Time Time evolution Wave Wave function
- Replies: 11
- Forum: Advanced Physics Homework Help
-

Graduate Coulombic interaction between the proton and electron of a hydrogen atom
In the position representation, its true that we can use operators to represent the coulombic interaction between the proton and electron of a Hydrogen atom. I've never actually given any thought as to what the elements of such an operator would be (in matrix form of course). I know these...- Demon117
- Thread
- Atom Electron Hydrogen Hydrogen atom Interaction Proton
- Replies: 2
- Forum: Quantum Physics
-
Hydrogen atom. state after L_z measurement
Homework Statement An initial state is given: {\lvert {\psi(0)} \rangle} = \frac{1}{{\sqrt{3}}} \left( {\lvert {100} \rangle} + {\lvert {210} \rangle} + {\lvert {211} \rangle}\right) An L_z measurement is performed with outcome 0 at time t_0. What is the appropriate form for the ket...- Peeter
- Thread
- Atom Hydrogen Hydrogen atom Measurement State
- Replies: 2
- Forum: Advanced Physics Homework Help
-
M
Why does helium have a greater first ionization energy than hydrogen?
I have had this question in the back of my mind for a while. Hydrogen has 1 proton and 1 electron, and the atom is electrically neutral. So that means that the electrical charge from the electron and the proton cancel each other out as they have equal/opposite charge. Then when you add another... -
T
Graduate Number of electron of a Hydrogen atom (molecule)
Hi, If the normalized 1D wave-function of hydrogen atom for n=1, l=0, m_l=0; \psi_{1s}(x)=\frac{1}{\sqrt{\pi} a_{0}^{3/2}}e^{-x/a_{0}} and probability distribution of wave-function, \mid\psi_{1s}(x)^2\mid so integration of rho over all x should give the number of electrons which is equal to...- torehan
- Thread
- Atom Electron Hydrogen Hydrogen atom Molecule
- Replies: 8
- Forum: Quantum Physics
-
M
Hydrogen Atom Tables: Find Zeros of Spherical Bessel Function
I've searched google for some decent tables but couldn't find any. I'm trying to collect Normalized Spherical Harmonics Associated Legendre Polynomials Zeros of the Spherical Bessel Function Normalized Radius Function for the Hydrogen atom etc. I'm allowed a sheet with as much of these...- Matthollyw00d
- Thread
- Atom Hydrogen Hydrogen atom
- Replies: 1
- Forum: Advanced Physics Homework Help
-

Graduate Fusion of the isotopes of hydrogen
Hi I assume that two hydrogen atoms fuse together to form one helium atom during nuclear fusion. But what happens when other isotopes of hydrogen fuse together? What happens to two deuterium atoms when they fuse together? Tritium? I'm assuming that they would form an isotope of helium, but... -
S
Graduate Can anyone confirm the ground state Bohr radius for muonic hydrogen?
Thanks in advance for anybody who is kind enough to help me. No this isn't for my homework. I am not even enrolled in school. I am doing some calculations for personal research. But I need to know the ground state radius of a muonic hydrogen atom to help prove my theory. I already know the...- seattle.truth
- Thread
- Bohr Bohr radius Ground Ground state Hydrogen Radius State
- Replies: 1
- Forum: Quantum Physics
-
P
Matrix element of Position Operator For Hydrogen Atom
Find <nlm|(1/R)|nlm> for the hydrogen atom (nlm = 211), R is just the radial position Operator (X2 + Y2 + Z2)½.- physics2004
- Thread
- Atom Element Hydrogen Hydrogen atom Matrix Operator Position Position operator
- Replies: 1
- Forum: Advanced Physics Homework Help
-
G
Graduate Solving the Dirac Equation for the Hydrogen Atom
Would someone tell me some website where I can find the relativistic treatment of the hydrogen atom using Dirac's Equation? I am not trying the find the method which uses Schrödinger's equation and adds as perturbations fine and hyperfine structures? Thank you. So far i have not find anything...- go quantum!
- Thread
- Atom Dirac Dirac equation Hydrogen Hydrogen atom
- Replies: 2
- Forum: Quantum Physics
-
M
What are the inconsistencies and errors in common measurement units?
i know the rules of increasing and decreasing atom sizes down and across the periodic table. however, i have met one strange thing when i was given data to plot a graph. the hydrogen atom radius was stated as 32 pm, but helium was 50 pm. when i searched the internet, i found few websites... -
A
Graduate The Hydrogen Atom (Schrodinger vs original Bohr theory)
What quantities appear in both the Schrödinger approach and the original Bohr theory of a hydrogen atom? Also, in what ways do the two approaches differ? I'm not sure which quantities appear in both approaches. My guess would be that it has something to do with the energy levels and angular...- aheizler
- Thread
- Atom Bohr Hydrogen Hydrogen atom Theory
- Replies: 1
- Forum: Quantum Physics
-
M
Lowest Energy State of Hydrogen Atom: Find A, Don't Miss the Obvious!
A hydrogen atom is in the state \psi=Ar^2e^{-r/a}cos(\theta). I need to find lowest energy state and etc. Obviously normalize to find A, but I'm not seeing the obvious linear combination of wave functions; and I really don't think my instructor wants me to do several inner products (plus...- Matthollyw00d
- Thread
- Atom Hydrogen Hydrogen atom State
- Replies: 5
- Forum: Advanced Physics Homework Help
-
S
Can Hydrogen be made from air and water?
Is forming ammonium nitrate and hydrogen from air and water is possible. N2+3H2O > NH4NO3 +H2 Is this reaction exothermic? Ammonium nitrate has enthalpy of formation of -365.1- sr241
- Thread
- Air Hydrogen Water
- Replies: 9
- Forum: Materials and Chemical Engineering
-
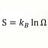
Newtons Law of Cooling applied to hot Neon and Hydrogen gas
Homework Statement 1 mole of Hydrogen gas at 300 Kevin is contained in a thin walled copper container. In container #2 there is 1 mole of Neon gas also at 300 Kevin. The volume= 22.4 liters and surface area ,A = .476 m^2 The surroundings are at 100 deg Kelvin. The specific heat of H2= 5...- Point Conception
- Thread
- Applied Cooling Gas Hot Hydrogen Law Neon Newtons Newtons law
- Replies: 6
- Forum: Biology and Chemistry Homework Help
-
D
Solving the Mystery of Hydrogen Atom Ionization
Here comes a pretty hard question, which not even my QM teacher has been able to answer. When we think about one hydrogen atom, and put it in an electric field along the z-axis \bar E = \bar e_z E. Then the potential for a hydrogen atom will look like this: U = -\frac{e^2}{4\pi \epsilon_0...- dreamspy
- Thread
- Atom Hydrogen Hydrogen atom Ionization Mystery
- Replies: 10
- Forum: Advanced Physics Homework Help
-
H
Proving the Visible Emissions of Balmer Series in Hydrogen Atom
Hello, I don't know if this is the right place to post.. this is a self study question from Levine, it is told that is important but i have no clue in solving it .. The Balmer series corresponds to a set of emissions involving the electron in a hydrogen atom relaxing from a high energy...- heavenchemist
- Thread
- Atom Emissions Hydrogen Hydrogen atom Series
- Replies: 6
- Forum: Advanced Physics Homework Help
-
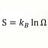
Cooling rates for Hydrogen and Neon gas
Homework Statement 1 mole of Hydrogen gas at 300 Kelvin is in a thin walled copper container vol 22.4 liters surface area A = .476 M^2 In vessel #2 , 1 mole of Neon gas also 300 Kevin The specific heat of H2= 5 cal/mole (K) Thermal conductivity (k) H2 = .0433 cal/sec. converts to .00866...- Point Conception
- Thread
- Cooling Gas Hydrogen Neon
- Replies: 1
- Forum: Introductory Physics Homework Help
-
L
What characteristic of a base enables it to ''accept'' Hydrogen Ions?
According to the Brønsted-Lowry definition, a base is a compound that can -keyword- accept H+, and an acid anything that can give H+.- LogicalAcid
- Thread
- Base Characteristic Hydrogen Ions
- Replies: 2
- Forum: Chemistry
-
C
Graduate About the unit of Radial wave function R(r) of Hydrogen atom
The 1s radial function of the wave function of H atom is: R10=2 a-3/2e-r/a ,where a = 5.29*10-11 meter but substituting a with its value,we will get R10 = 5.2*1015 *e(-1.89036*1010 r) and that is impossible if r=a and R(r)=1.9*1015 where is the problem ? What's more, the unit...- caoyuan9642
- Thread
- Atom Function Hydrogen Hydrogen atom Radial Unit Wave Wave function
- Replies: 5
- Forum: Quantum Physics
-
C
Undergrad Is an Electron a Boson or a Fermion?
I'm studying particle physics now and am confused with all these terminologies.- Chaste
- Thread
- Atom Fermion Hydrogen Hydrogen atom
- Replies: 6
- Forum: Quantum Physics
-
P
Graduate Muonic hydrogen ground state energy
Would the energy just be a multiple of how much bigger it is than electron?- petey_hb69
- Thread
- Energy Ground Ground state Ground state energy Hydrogen State
- Replies: 2
- Forum: High Energy, Nuclear, Particle Physics
-
B
First-order time-dependent perturbation theory on a Hydrogen atom
Homework Statement A Hydrogen atom is initially in its ground state and then subject to a pulsed electric field E(t)=E_{0}\delta(t) along the z direction. We neglect all fine-structure and hyperfine-structure corrections. Homework Equations 1. It is important to use selection rules to avoid...- boyu
- Thread
- Atom Hydrogen Hydrogen atom Perturbation Perturbation theory Theory
- Replies: 1
- Forum: Advanced Physics Homework Help
-
B
Graduate Allowed Transition from 2s^2 to 2p^2 in Hydrogen: Why is it Missing?
Why is there no allowed transition from the 2s^2 S_{\frac{1}{2}} state to the 2p^2 P_{\frac{3}{2}} state in the attached image? It seems to fulfill the selection rules \Delta l = \pm 1 and \Delta j = 0, \pm 1. This is for electric dipole transitions by the way.- barnflakes
- Thread
- Hydrogen Rules Selection rules
- Replies: 1
- Forum: Atomic and Condensed Matter
-
U
Calculate Transition in Hydrogen for 600nm Wavelength
Homework Statement use the RYDBERG formula to suggest a possible transition that would result in the observed wavelength Homework Equations Hydrogen - yellow wavelength about 600nm The Attempt at a Solution I used c= lambda x frequency(V) and found (V) to equal 5e14/second I then...- unf0r5ak3n
- Thread
- Hydrogen Transition
- Replies: 7
- Forum: Biology and Chemistry Homework Help
-
M
Add carbon atom to pure hydrogen
Looking for ideas that allow me to create Methane ( CH4 ) from gaseous hydrogen. This requires bonding one carbon atom to four hydrogen atoms, which should be simple enough, but there needs to be a minimum explosive danger. I know there are appliances to go the other way, but don't have the... -
N
Calculating Rotational & Vibrational Energy of Hydrogen Molecule
Homework Statement The potential energy V between the two hydrogen atoms ( mH = 1.672*10^(-24) g) in a hydrogen molecule is given by the empirical expression V = V0 [ exp(-2a(r-r0)) - 2exp(-a(r-r0))] where V0 = 7*10^(-12) erg, a = 2*10^8 cm-1, r0 = 8*10^-9 cm. (a) Estimate the...- nicky04
- Thread
- Energy Hydrogen Molecule Rotational
- Replies: 6
- Forum: Advanced Physics Homework Help
-
M
Graduate Ionizing solutions to the hydrogen atom
Are there any ionizing solutions for the hydrogen atom problem, where the electron breaks away from the proton?- mordechai9
- Thread
- Atom Hydrogen Hydrogen atom
- Replies: 5
- Forum: Quantum Physics