Process Definition and 1000 Threads
-
F
Auto-covariance of a Wiener process of a function
Homework Statement Let {W(t); t >= 0} be a Wiener process. Determine the auto-covariance function for the process {X(t); t >= 0} defined by X(t) = e^(-ct) * W(e^(2ct)) for all t >= 0, where c > 0 is a constant. Is {X(t); t >= 0} stationary in the wide sense? Homework Equations Is...- fortissimo
- Thread
- Function Process
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
D
Show that the Gram-Schmidt process gives the shortest length
Homework Statement Let ##v_{1},...,v_{m}## be an orthonormal set of vectors in ##V##. Let ##v\not\in S(v_{1},...,v_{m})##. Show that the vector ##v'=v-\sum\limits_{i=1}^{n}(v,v_{i})v_{i}## given by the Gram-Schmidt process has the shortest length among all vectors of the form ##v-x## for ##x\in...- DeadOriginal
- Thread
- Length Process
- Replies: 7
- Forum: Calculus and Beyond Homework Help
-

Tips for Buying a Home""Buying a Home: Tips to Make the Process Easier
- D H
- Thread
- Home Process Tips
- Replies: 12
- Forum: STEM Career Guidance
-
C
Viscous fluid flow in a manufacturing process
In work that I've been doing, we've come across an interesting problem. In a manufacturing process, a highly viscous, non-Newtonian fluid goes down a vertical chute and then gets pushed horizontally out the bottom by some rotating screws. Sometimes, the fluid gets pushed out the bottom, but...- czechman45
- Thread
- Flow Fluid Fluid flow Manufacturing Process viscous
- Replies: 2
- Forum: Mechanical Engineering
-
H
MHB Probability that all N_Q packets arrived in [0,t], in a Poisson process
Arrivals are Poisson distributed with parameter $$ \lambda$$. Consider a system, where at the time of arrival of a tagged packet, it sees $$N_Q$$ packets. Given that the tagged packet arrives at an instant $$t$$, which is uniform in [0, T], what is the probability that all $$N_Q$$ packets...- hemanth
- Thread
- Poisson Poisson process Probability Process
- Replies: 1
- Forum: Set Theory, Logic, Probability, Statistics
-
A
Graduate Adiabatic Process: C_v ≠ 0 Despite dQ = 0
The low is: dQ=dU+p dV but the specific heat to volumen (in a perfect gas) cte is: C_v = \frac{dQ}{dT} = \frac{dU}{dT} why if in adiabatic proces dQ=0, then C_v \neq 0 ?? http://en.wikipedia.org/wiki/Adiabatic_process#Ideal_gas_.28reversible_process.29- alejandrito29
- Thread
- Adiabatic Adiabatic process Process
- Replies: 2
- Forum: Thermodynamics
-
Z
Undergrad Solving Probability of Process: Sketching Functions & Annual Savings
Hi, I'm trying to solve the following question, but unsure how to approach it. A paper manufacturing process states (in its specifications) that each piece of paper's weight will be less than the nomial weight of 1.2 grams on NO MORE than 1 occasion in 100. Currently, the process produces to...- zaka
- Thread
- Probability Process
- Replies: 1
- Forum: Set Theory, Logic, Probability, Statistics
-
C
Undergrad Work done by gas in adiabatic process
In an adiabatic process PVγ=constant Now I thought the work done by an ideal gas in an adiabatic process was given by the equation here: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/adiab.html But while doing a GRE question the answer was given as PfVf - PiVi / 1 - γ Is this...- collectedsoul
- Thread
- Adiabatic Adiabatic process Gas Process Work Work done
- Replies: 3
- Forum: Mechanics
-
P
Graduate Nuclear Fusion process in the Sun (or generally, any star)
Why can't the Sun (or any star) fuse elements higher than iron? Could anyone provide a technical answer? Thanks!- Positron137
- Thread
- Fusion Nuclear Nuclear fusion Process Star Sun The sun
- Replies: 18
- Forum: Astronomy and Astrophysics
-
C
Undergrad Thought process improvement on Probabilities
I had a problem that said "I throw nine balls randomly in five containers. What's the probability any container will have exactly five balls by the end of the process?" The answer involved using a Binomial distribution. Now the question is, how do I train my mind to go to the use of...- cdux
- Thread
- Probabilities Process
- Replies: 3
- Forum: Set Theory, Logic, Probability, Statistics
-
R
Thermodynamic process P^2+aV^2=constant
Homework Statement One mole of ideal gas expands from an initial volume V1,to a final volume V2. Where V1 and P1 correspond to the volume and initial gas pressure, whereas V2 and P2 correspond to the volume and pressure at the end of the process. The process is described by the equation...- rafaelgm9
- Thread
- Process Thermodynamic
- Replies: 1
- Forum: Introductory Physics Homework Help
-
I
Is it important to enjoy the process of doing physics?
Apologies if this has been asked before but I couldn't find any posts with the exact question. I'm about to go to college but I haven't decided my major yet. I have loved physics, particularly astronomy, ever since I was a child. I used to read a lot of astronomy books when I was younger...- Ilikecereal
- Thread
- Important Physics Process
- Replies: 7
- Forum: STEM Career Guidance
-
C
Work done in thermodynamic process
Homework Statement An ideal gas undergoes the following reversible cycle: (a) an isobaric expansion from (P1, V1) to (P1, V2), (b) an isochoric reduction from (P1, V2) to (P2, V2) (c) an isobaric reduction from (P2, V2) tp (P2, V1) (d) an isochoric expansion from (P2,V1) to (P1, V1). What...- CAF123
- Thread
- Process Thermodynamic Work Work done
- Replies: 18
- Forum: Introductory Physics Homework Help
-
J
E and the wrong thought process for limits
Homework Statement So I keep evaluating these limits wrong. Something is wrong in my thought process. Homework Equations http://www.calcchat.com/book/Calculus-ETF-5e/ chapter 9, section 1, question 69 Consider A_n = (1+k/n)^n If I wanted to see if it converged or not I would just...- Jbreezy
- Thread
- Limits Process
- Replies: 17
- Forum: Calculus and Beyond Homework Help
-
D
Stochastic Process prerequsites and difficulty?
My university is offering a course called "Stochastic Process". The only prerequisites to this course according to my university is a course in Probability which uses the book by Rosen. I've read elsewhere that the course actually requires more of analysis (functional analysis and measure...- Dens
- Thread
- Difficulty Process Stochastic Stochastic process
- Replies: 1
- Forum: STEM Academic Advising
-
D
Why Is the Process Epsilon Meson to Eta Meson Forbidden?
Does anyone have any idea why the process epsilon meson to eta meson is forbidden? bb' --> cc' Mass Energies 9.3 GeV --> 3.0 GeV The solution set simply says that this is kinematically forbidden. It seems to me that in the center of momentum frame the products can have whatever...- dsdsuster
- Thread
- Process
- Replies: 1
- Forum: Advanced Physics Homework Help
-
S
Graduate Ito integral of an arbitrary f(Wiener process)
How would you take the Ito integral of an arbitrary f(W_T) where W_T is a standard Wiener process X_T=\int^t_0 f(W_s)dW_s would you somehow use Ito's lemma? I have attempted, but it doesn't seem to make sense... dX_T=f(W_T)dW_T, There doesn't seem to be a f(x) that makes sense for this in...- saminator910
- Thread
- Integral Process
- Replies: 1
- Forum: Calculus
-
I
Graduate Photons Impact on the Visual Process
My goal is to build a software program that simulates the function of the eye. As I (barely) understand things, a single photon can bounce off an object and enter my eye. Q1 - Does the photon determine the color? If not, what determines the color of that single wave/particle? Q2 - How...- iowahawk43
- Thread
- Impact Photons Process Visual
- Replies: 7
- Forum: Quantum Physics
-
A
What is the Maximum Pressure in kPa for Ammonia in the Haber Process?
Hello everyone, I'm doing some practice problems for my final exam. I came across this question: «The Haber process is being used at a pressure of 76 000 mmHg at a temperature of 450°C. Gaseous ammonia is cooled and collected after its production, in order that its temperature does not...- alingy1
- Thread
- Gas Gas laws Laws Process
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
D
Can You Give Examples of Industrial Processes That Depend on Gas Pressure?
Hello can someone please give me an example of TWO industrial process which uses and depends on gas pressure or the change in gas pressure. Basically did this but instead wrote about gas Laws uses in different things so have to re do it (didn't read the question as we were learning about gas...- dee 123
- Thread
- Example Industrial Process
- Replies: 14
- Forum: Introductory Physics Homework Help
-
M
Thermodynamics Isochoric process .
Hi can please check my answer for this question : A certain Gas of volume 0.4 m3, pressure of 4.5 bar and temperature of 1300 C is heated to in a cylinder to 9 bar when the volume remains constant. Calculate ¨ (i) Temperature at the end of the process, ¨ (ii) the heat...- manal950
- Thread
- Isochoric Process Thermodynamics
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
B
Graduate Properties of adiabatic process
Hello Can we consider the sentence “Adiabatic process cannot decrease entropy” (found for example here) to be true in any circumstances ? Here I do not want to focus on any particular situation. Hence the system is not necessarily a (perfect) gas; the transformation is not necessarily...- burakumin
- Thread
- Adiabatic Adiabatic process Process Properties
- Replies: 24
- Forum: Thermodynamics
-
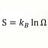
Entropy For Irrevesible Process
Homework Statement Two 100g Aluminum cubes with T1 = 80° C and T2 = 20°C Specific heat of Al is .22 cal/g/°C. The cubes are placed in contact and in short period of time a quantity of heat, dQ , is transferred from T1 cube to T2 cube. The cubes temperatures now are 75°C and 25°C. After a...- Point Conception
- Thread
- Entropy Process
- Replies: 9
- Forum: Introductory Physics Homework Help
-
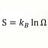
Graduate S2 - S1 > Integral 1-2 dQ/T Irreversible Process. What T ?
For an irreversible process: S2 - S1 > ∫12 dQ/T In the case of heat transfer dQ between object with T1 to object with T2, T1 > T2 ΔS in Joules/K is known But for evaluating ∫12 dQ/T = ln dQ/T - ln dQ/T . d/Q is known but what values for T in this case ? Object 1 & 2 have Temperatures before...- Point Conception
- Thread
- Integral Irreversible Process
- Replies: 6
- Forum: Thermodynamics
-

What is the Efficiency of a Cyclic Process?
Homework Statement (see attachment, ignore the arrows made with the pen) Homework Equations The Attempt at a Solution Efficiency of a cycle is defined as ##\eta=\frac{W}{Q}## where W is work done and Q is heat input. W can be easily calculated by finding the area enclosed...- Saitama
- Thread
- Cyclic cyclic process Efficiency Process
- Replies: 6
- Forum: Introductory Physics Homework Help
-
M
"Understanding Random Process X(t) and Its Sample Realizations
Problem statement: Define the random process X(t) = C where C is uniform over [-5,5]. a) Sketch a few sample realizations I need reassurance that if I do a a few sample realizations of this random process they are all going to look the same. They are going to be an horizontal line with...- marina87
- Thread
- Process Random Random process
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
D
Graduate Electrostatics in RC\DC Circuits-feedback process
Hello, I've always had problems conceptualizing the physics behind circuits, and it always felt like information is hidden from me. Lately I've been trying to analyze circuits in the microscopic and electrostatic way, as i think it is crucial for real understanding of circuit concepts. I've...- Dweirdo
- Thread
- Electrostatics Process
- Replies: 2
- Forum: Electromagnetism
-
J
MHB Optimizing Least Square Method for Ill-Conditioned Linear Problems
Hi does anyone know the "formula/process" for the least square method if the are giving several (x,y) points and asking me to find y=Ax^2 + B?- Juliayaho
- Thread
- Method Process Square
- Replies: 8
- Forum: Topology and Analysis
-
E
Graduate The average of a random process
Hello all, I have the following continuous-time random process: v(t)=\sum_{k=0}^{K-1}\alpha_k(t)d_k+w(t) where d_k are i.i.d. random variables with zero mean and variance 1, alpha_k(t) is given, and w(t) is additive white Gaussian process of zero-mean and variance N_0. Can we say...- EngWiPy
- Thread
- Average Process Random Random process
- Replies: 3
- Forum: Set Theory, Logic, Probability, Statistics
-
K
Question regarding non flow steam process
Hi There, quite an informative forum.Im struggling with a question which reads as follows: At a certain point during the expansion cycle in a steam engine cylinder, the steam pressure was 1.1 MPa and the dryness 0.85, At the end of the expansion process the pressure was 0.28Mpa and the dryness...- kanzeon1
- Thread
- Flow Process Steam
- Replies: 2
- Forum: Engineering and Comp Sci Homework Help
-
M
Is the Angular Equation Divided by Radius in This Example?
attached is an example question i am working through, i would appreciate if anybody could explain to me the part in the red box. I understand the relationship linear acceleration = ang acceleration*radius however, this working appears to divide the angular equation by the radius? thanks- MMCS
- Thread
- Process
- Replies: 3
- Forum: Introductory Physics Homework Help
-
S
Renewal Process: laplace transform approach
Homework Statement Use the Laplace transform approach to find the renewal function for a renewal process with interrenewal p.d.f. as follows: g(x) = (c^2)xe^(-cx) , x > 0 The Attempt at a Solution M*(s) = G*(s)/(1-G*(s)) where M*(s) and G*(s) denote laplace transforms I have that G*(s) =...- SantyClause
- Thread
- Approach Laplace Laplace transform Process Transform
- Replies: 3
- Forum: Calculus and Beyond Homework Help
-
P
What is the role of zero-momentum frame in two-photon collision processes?
Homework Statement Homework Equations Zero-momentum frame is defined as the frame in which total momentum is zero. The Attempt at a Solution (a) In terms of the zero momentum variables, (e1 and e1 are to distinguish the two resultant particles) \vec{p'}_{HE} +...- Pi-Bond
- Thread
- Collision Process
- Replies: 17
- Forum: Advanced Physics Homework Help
-
A
Graduate Irreversible process Entropie-Change/cylinder
Hi, I have read that for an irreversible process the equation for the entropy is: $$ds>\frac{dQ}{T}$$ But if I regard a cylinder connected to a heatreservoir and want to callculate the entropychange of this cylinder, why can I use the equation: $$ds=\frac{dQ}{T}$$? THX- Abigale
- Thread
- Irreversible Process
- Replies: 1
- Forum: Thermodynamics
-
W
Entropy change in isochoric process
Homework Statement Hi everyone In an isochoric process ( a solid heat body with T1 temp. connected with heat bath with T2 temp) we could calculate its entropy change(heat body) from dS = Cv/T dT and entropy change of heat bath from dS = dQ / T2, using dQ = Cv dT in 1st equation ( i...- wowowo2006
- Thread
- Change Entropy Isochoric Process
- Replies: 1
- Forum: Introductory Physics Homework Help
-
G
Schools European university masters program admission process
Looking at some German/Swiss masters programs (mainly computational mechanics in Stuttgart, München, Lausanne) but having a tough time figuring out the admission process for international students. Do they generally only look at your bachelor's degree GPA and nothing else or does other stuff...- Gauss M.D.
- Thread
- Admission Masters Process Program University
- Replies: 9
- Forum: STEM Academic Advising
-
J
Poisson Process Conditional Distribution
Homework Statement X_t and Y_t are poisson processes with rates a and b n = 1,2,3...Find the CDF F_X{}_t{}_|{}_X{}_t{}_+{}_Y{}_t{}_={}_n(x)Homework Equations The Attempt at a Solution F_X{}_t{}_|{}_X{}_t{}_+{}_Y{}_t{}_={}_n(x) =P(X_t<x|X_t+Y_t=n) =\frac{P(X_t<x,X_t+Y_t=n)}{P(X_t+Y_t=n)} Not...- jiml
- Thread
- Conditional Distribution Poisson Poisson process Process
- Replies: 4
- Forum: Calculus and Beyond Homework Help
-
E
Graduate Does this type of process have a name?
E(Xn+1+i)=Ʃn+ij=i+1 cj-iXj, where Ʃnj=1 cj = 1. n is a fixed constant here, c is a fixed set of n coefficients. Can anyone tell me anything about such a process?- euroazn
- Thread
- Process Type
- Replies: 6
- Forum: Set Theory, Logic, Probability, Statistics
-
A
Graduate What is the relationship between entropy and the irreversibility of a process?
Hi, I regard a irreversible cycle process. It is a cylinder filled with gas, which underlies the following processes: 12: isothermal compression (reversible) 23: adiabatic expansion (reversible) 31: isochor heating (irreversible) [This happens by connecting the cylinder with a...- Abigale
- Thread
- Irreversible Process
- Replies: 2
- Forum: Thermodynamics
-
N
Chemistry Chemical Process Analysis - Mole Flow Rate
Homework Statement 120 mol/min of Propane (C3H8) is burned in the presence of air (21% O2 and 79% N2) in a furnace, two reactions occur: Complete Combustion: 67%, propane is burned to CO2 and H2O Incomplete Combustion: 18%, propane is burned to CO and H2O Oxygen is supplied at 70%...- nobodyuknow
- Thread
- Analysis Chemical Flow Flow rate Mole Process Rate
- Replies: 2
- Forum: Biology and Chemistry Homework Help
-
X
Galvanizing: A Surface Treatment?
For the basis of a report in university, i have been given an assignment which involves discussing various surface treatments. Is galvanizing a surface treatment? Some sources refer to it as a surface coating because obviously it involves building up layers of zinc oxide.- Xander_11
- Thread
- Process
- Replies: 2
- Forum: Materials and Chemical Engineering
-
C
Graduate Is Conservation of Energy Sufficient for Deriving the Adiabatic Process?
Most of the book using PV=nRT for PV^gamma constant for adiabatic process. However, please refer attachment, proving using conservation of energy will also make sense?- chuakoktong
- Thread
- Adiabatic Adiabatic process Derivation Process
- Replies: 10
- Forum: Thermodynamics
-
P
Identify process in a heat engine
Homework Statement I'm taking a practice test and I need some help with a heat engine problem. There are three parts, the one that's giving me trouble is a-->b. The Volume v. Pressure graph shows a straight line. Pa= 5 atm Va= 5 L Ta=300K and Tb=800K are given. So pressure is decreaseing...- physics.stu
- Thread
- Engine Heat Heat engine Process
- Replies: 5
- Forum: Introductory Physics Homework Help
-
M
Graduate Random process derived from Markov process
I have a query on a Random process derived from Markov process. I have stuck in this problem for more than 2 weeks. Let r(t) be a finite-state Markov jump process described by \begin{alignat*}{1} \lim_{dt\rightarrow 0}\frac{Pr\{r(t+dt)=j/r(t)=i\}}{dt} & =q_{ij} \end{alignat*} when i \ne...- Mubeena
- Thread
- Markov process Process Random Random process
- Replies: 4
- Forum: Set Theory, Logic, Probability, Statistics
-
F
Undergrad Idea gas law applied to throttling process
Hi all. I'm trying to become acquainted with thermal dynamics. I thought I had a fairly good handle on the idea gas law, PV=nRT, or PV=mRT, but I came across a problem involving the throttling of an idea gas, and the solution was totally not what I expected. Referring to picture where gas...- FrankJ777
- Thread
- Applied Gas Gas law Idea Law Process
- Replies: 7
- Forum: Thermodynamics
-
M
Fischer–Tropsch process and catalysts?
I want to know what catalyst is used in the Fischer–Tropsch process to get fuels. -
5
Process for finding a definite integral
Homework Statement given that [SIZE="4"]∫01 xndx = 1/ n+1 for integers n \geq 0 calculate these integrals Homework Equations [SIZE="4"]∫01 (1+x+x2) dx The Attempt at a Solution I have found the definite integral of [SIZE="4"]∫01 (1+x+x2) dx to = 1.8333... (ie. 11/6) but all I...- 5ymmetrica1
- Thread
- Definite integral Integral Process
- Replies: 6
- Forum: Calculus and Beyond Homework Help
-
S
Poisson Process: interevent times
Homework Statement Consider a one-way road where the cars form a PP(lambda) with rate lambda cars/sec. The road is x feet wide. A pedestrian, who walks at a speed of u feet/sec, will cross the road if and only if she is certain that no cars will cross the pedestrian crossing while she is on...- SantyClause
- Thread
- Poisson Poisson process Process
- Replies: 1
- Forum: Calculus and Beyond Homework Help
-
P
Question regarding Rolling process
In a rolling process, the state of stress of the material undergoing deformation is a) pure compression b) pure shear c) compression and shear d) tension and shear Which one is the correct option ? Please explain why !- pranoy407
- Thread
- Process Rolling
- Replies: 4
- Forum: Mechanical Engineering
-

Revolutionary Process Reduces Coal Pollution by 99%
http://www.foxnews.com/science/2013/02/20/coal-cleanest-energy-source-there-is/?intcmp=HPBucket#ixzz2LUZ8Z69v I'm not seeing it. The problem is obvious: The primary combustion products of coal are water and carbon dioxide. The proportions vary with the type of coal, but a considerable...- russ_watters
- Thread
- Coal Pollution Process
- Replies: 13
- Forum: Materials and Chemical Engineering