Discussion Overview
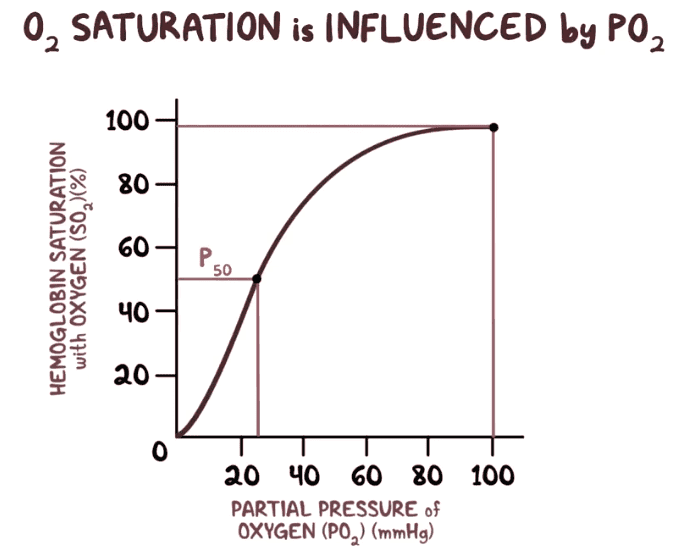
The discussion revolves around the binding of oxygen (O2) to hemoglobin (Hb) molecules, focusing on the relationship between partial pressure of O2 and hemoglobin saturation. Participants explore the implications of the sigmoidal binding curve, cooperative binding, and the interpretation of binding affinity at varying saturation levels.
Discussion Character
- Exploratory
- Technical explanation
- Debate/contested
- Mathematical reasoning
Main Points Raised
- Some participants note that the binding capacity of hemoglobin is proportional to the partial pressure of O2, but question the interpretation of this relationship as "directly proportional" due to the non-linear nature of the sigmoidal curve.
- There is a discussion about whether the statement regarding increasing affinity of Hb for O2 as more O2 binds is universally applicable or only relevant at low partial pressures.
- One participant emphasizes that hemoglobin has four oxygen binding sites that are not independent, highlighting the concept of cooperative binding where binding subsequent O2 molecules becomes "easier" due to conformational changes in the protein.
- Another participant expresses confusion about the implications of the sigmoidal curve, suggesting that it resembles an exponential curve initially but is affected by saturation, which reduces the slope as saturation increases.
- A later reply introduces the concept of equilibrium constants and critiques the use of the term "sigmoid," suggesting it can lead to confusion in understanding binding dynamics.
- Participants discuss the importance of distinguishing between cooperative and non-cooperative binding curves, noting that the interpretation of binding difficulty varies depending on the context of saturation and affinity.
Areas of Agreement / Disagreement
Participants express differing views on the interpretation of hemoglobin's binding curve and the implications of cooperative binding. There is no consensus on the clarity of the terms used or the best way to describe the relationship between saturation and binding affinity.
Contextual Notes
Some limitations in the discussion include potential misunderstandings of the terms "directly proportional" and "sigmoid," as well as the complexities involved in defining binding difficulty and equilibrium constants in the context of cooperative binding.