TjFitz
- 9
- 0
Hey ya,
Well, I have been having a difficult time finding the answer to this question, probably because I don't know the specific terminology. So here I am. I did not know exactly which forum category to put this in so...
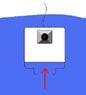
I am building a small diving bell with a remote controlled camer inside. Nothing more than a hobby project..
The Bell is actually nothing more than a 1 liter Pickle Jar.
What I am wondering is, how much compression of air can I expect at let's say a max of 10 meter depth. meaning if I have 1 liter of air in the jar, how far will that compress per meter fresh water depth.
I would like to know this because I need to know how much room in the jar I have that I can use, or even how deep I should limit myself before water would reach my gear in the jar.
is there a simple equation which states X cc/ml of air compression per meter depth?
Well, I have been having a difficult time finding the answer to this question, probably because I don't know the specific terminology. So here I am. I did not know exactly which forum category to put this in so...
I am building a small diving bell with a remote controlled camer inside. Nothing more than a hobby project..
The Bell is actually nothing more than a 1 liter Pickle Jar.
What I am wondering is, how much compression of air can I expect at let's say a max of 10 meter depth. meaning if I have 1 liter of air in the jar, how far will that compress per meter fresh water depth.
I would like to know this because I need to know how much room in the jar I have that I can use, or even how deep I should limit myself before water would reach my gear in the jar.
is there a simple equation which states X cc/ml of air compression per meter depth?