barnflakes
- 156
- 4
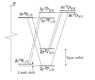
Why is there no allowed transition from the 2s^2 S_{\frac{1}{2}} state to the 2p^2 P_{\frac{3}{2}} state in the attached image? It seems to fulfill the selection rules \Delta l = \pm 1 and \Delta j = 0, \pm 1. This is for electric dipole transitions by the way.