rwooduk
- 757
- 59
I want to use a pump to have solution moving through a reaction vessel at a set flow rate. But the problem is the outlets FROM the pump are very small 1/6" (0.4cm) inner diameter, and the vessel INLET is GL14 thread size (1cm inner diameter).
Schematic
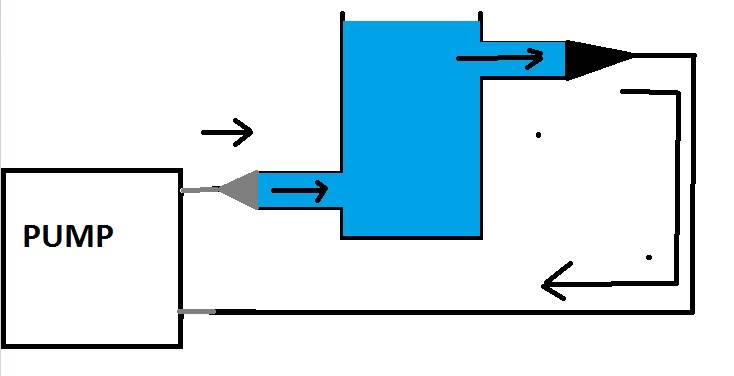
Problem
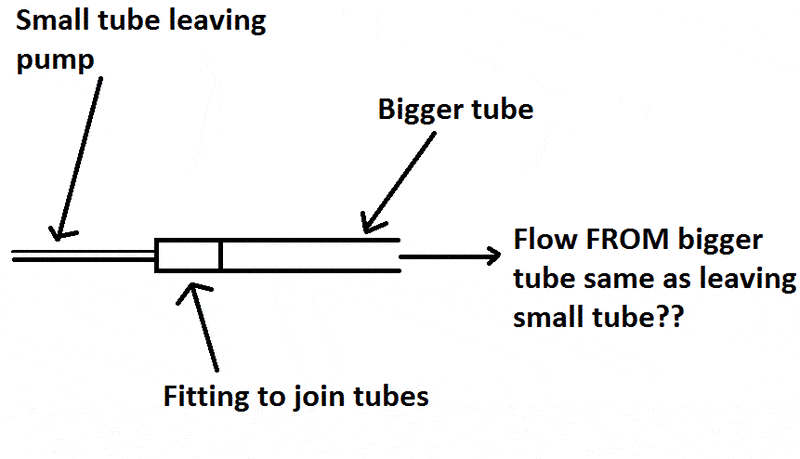
Pump
https://www.coleparmer.co.uk/i/mn/7316033
My question is will a change in tubing size effect the flow rate due to pressure? My initial thoughts are yes, however it is a closed system, so although there is pressure present as the pump "pushes" the liquid through it will after a short period of time equibrilate.
However when I visualise it the flowing fluid from the small tube will disperse into the big one and there will not be the same flow rate in the bigger tube.
Any thoughts on this?
Schematic
Problem
Pump
https://www.coleparmer.co.uk/i/mn/7316033
My question is will a change in tubing size effect the flow rate due to pressure? My initial thoughts are yes, however it is a closed system, so although there is pressure present as the pump "pushes" the liquid through it will after a short period of time equibrilate.
However when I visualise it the flowing fluid from the small tube will disperse into the big one and there will not be the same flow rate in the bigger tube.
Any thoughts on this?