mess
- 59
- 8
- TL;DR
- I am working on a campervan project that i intend to take far up north in extreme cold climates, as such ive sealed it very well with thick foam insulation. There is nearly 0 air flow between the outside of the van and the inside.
On one of my test trips it was very cold, i kept all the windows and vents closed, and by the morning i had a severe migrain.
My goal is to determine the ideal amount of air flow, in order to keep CO2 below 1000ppm and to minimize heat loss.
I calculated the following based off a similar post in this forum, and I am hoping this can be verified so I know that i am in the right ballpark and going in the right direction as far a solution.
Fresh air has about 0.04% (400ppm) co2, non ideal air has a high limit of 0.1% CO2 (<1000ppm). the difference is .06%.
in a van with typical sprinter dimensions that's about 500 cubic feet minus any things you might have added in your build, but ill say 500.
so 500 cuft * .06% = .3 cuft can be added in order to pass non ideal co2.
and 500 cuft * .26% = 1.3 cuft can be added before you start to get sick from co2. because 0.3% (co2 sickness concentration) - 0.04% (fresh) = .26% difference.
A single occupant adds about 12 cubic feet per day of co2, or about .5 cuft per hour of co2.
That means at best, a single occupant has less than an hour before CO2 levels become not ideal, and just under 3 hours before they might get headache, lack of concentration and sleepiness from too much co2.
now getting into fatal numbers.
500 cuft * 1% = 50 cuft can be added before you might die from co2. This will happen in 100 hours for a single occupant, that's about ~4 days, ~2 days for double, less than that with pets.
Next to figure out a solution through venting in air from the outside:
when the inside air reaches nearly 1000ppm (i have a sensor that controls a vent fan and valve that's attached to the roof of my van now), the vent will open and the fan will suck air from the outside, and mix it in with the return air going into my diesel heater.
Inside air:
500 cf @ 0.1% co2
air coming in from vent :
20cfm @ 0.4% co2
500 cf / 20 cfm = 25 minutes
in 25 minues the entire volume will have been diluted. with would bring it to 50% of the co2%, 500ppm.
therefore at 20cfm, .05% (500ppm) can be removed in 25 min
in one hour that's about ~1000ppm.
but one human is adding .5% (5000ppm) CO2 in one hour. Or 5x more than I am removing.
Therefore in order to maintain around 500ppm in the van i need a flow rate of 20cfm*5 or 100cfm.
And to maintain around 1000ppm CO2 I need a flow rate of about 50cfm? (I think i might have done this part wrong)
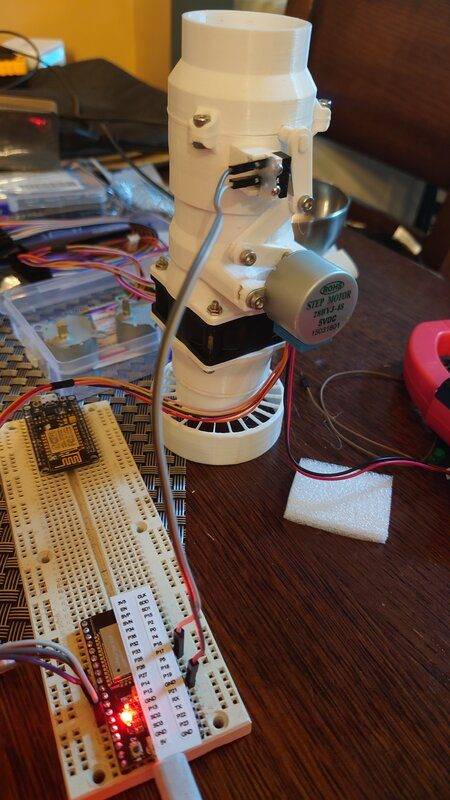
(pic of my 3d printed contraption)
Any help appreciated! thank you!
I calculated the following based off a similar post in this forum, and I am hoping this can be verified so I know that i am in the right ballpark and going in the right direction as far a solution.
Fresh air has about 0.04% (400ppm) co2, non ideal air has a high limit of 0.1% CO2 (<1000ppm). the difference is .06%.
in a van with typical sprinter dimensions that's about 500 cubic feet minus any things you might have added in your build, but ill say 500.
so 500 cuft * .06% = .3 cuft can be added in order to pass non ideal co2.
and 500 cuft * .26% = 1.3 cuft can be added before you start to get sick from co2. because 0.3% (co2 sickness concentration) - 0.04% (fresh) = .26% difference.
A single occupant adds about 12 cubic feet per day of co2, or about .5 cuft per hour of co2.
That means at best, a single occupant has less than an hour before CO2 levels become not ideal, and just under 3 hours before they might get headache, lack of concentration and sleepiness from too much co2.
now getting into fatal numbers.
500 cuft * 1% = 50 cuft can be added before you might die from co2. This will happen in 100 hours for a single occupant, that's about ~4 days, ~2 days for double, less than that with pets.
Next to figure out a solution through venting in air from the outside:
when the inside air reaches nearly 1000ppm (i have a sensor that controls a vent fan and valve that's attached to the roof of my van now), the vent will open and the fan will suck air from the outside, and mix it in with the return air going into my diesel heater.
Inside air:
500 cf @ 0.1% co2
air coming in from vent :
20cfm @ 0.4% co2
500 cf / 20 cfm = 25 minutes
in 25 minues the entire volume will have been diluted. with would bring it to 50% of the co2%, 500ppm.
therefore at 20cfm, .05% (500ppm) can be removed in 25 min
in one hour that's about ~1000ppm.
but one human is adding .5% (5000ppm) CO2 in one hour. Or 5x more than I am removing.
Therefore in order to maintain around 500ppm in the van i need a flow rate of 20cfm*5 or 100cfm.
And to maintain around 1000ppm CO2 I need a flow rate of about 50cfm? (I think i might have done this part wrong)
(pic of my 3d printed contraption)
Any help appreciated! thank you!
Last edited by a moderator: