Zhelives
- 1
- 0
I'm given the following matrix equation:
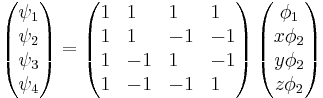
I'm also given:
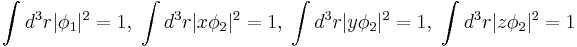
We're supposed to show that the angle between any two of these wave functions is 109.5 (characteristic of sp3 hybridization). I assume I need to calculate the expectation values of x, y and z and then use the dot product, but I'm at a loss on how to evaluate the inegrals. Suggestions?
Thanks.
P.S. Forum Question: Why do the images appear as links when I use the [\IMG] tags?
I'm also given:
We're supposed to show that the angle between any two of these wave functions is 109.5 (characteristic of sp3 hybridization). I assume I need to calculate the expectation values of x, y and z and then use the dot product, but I'm at a loss on how to evaluate the inegrals. Suggestions?
Thanks.
P.S. Forum Question: Why do the images appear as links when I use the [\IMG] tags?