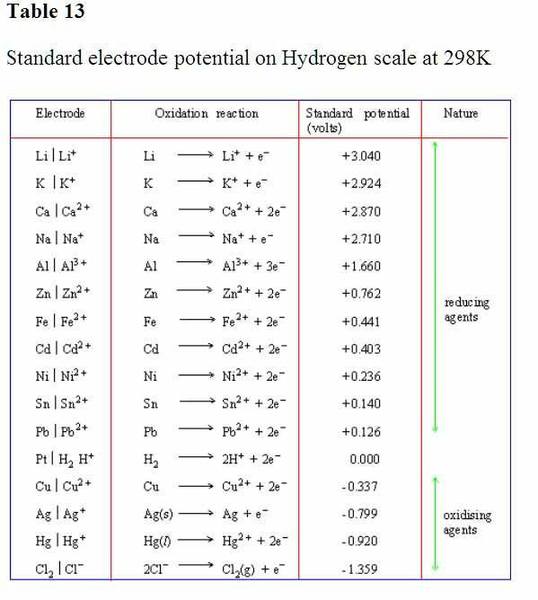
This is a classical problem in electrochemisty. You can find other tables of standard potentials, E°s, of half cell reactions having the same numerical value but opposite sign, for the reactions in your table . For example for the Li/Li+ -3.040V. Thus, every reaction in your table and that table will have opposite signs for the values of E°s. Therefore, the question arises whether the potential of LI/Li+ is +3.040V or -3.040V? The American tables give the positive value and the British tables give the negative value. Much can be said in support of each. This issue was resolved by an international convention, which uses the negative value for this half cell E°. Without going into too much of a detail, I shall be brief.
The way to understand the relation between E° and the ΔG° and the corresponding spontaneity is this way: Choose a reaction from the table. Combine it with H/H+ reaction. You will get a voltaic cell. A spontaneous reaction, in principle, is possible. Take for example, the Li/Li+ and couple it with SHE. You will get a cell with a E° value of 3.040V. In this cell Li goes to Li+ spontaneously and H+ goes to H2 spontaneously.
Now take, Cu/Cu2+ reaction. Combine it with H/H+ reaction. You will get a voltaic cell. A spontaneous reaction, in principle, is possible. You will get a cell with a E° value of 0.337V. In this cell H2 goes to H+ spontaneously and Cu2+ goes to Cu spontaneously.
Now you can think of combining Li/Li+ and Cu/Cu2+ reactions (or any two reactions for that matter). You get a cell with a E° value of the difference of the two E° values, 3.377V. In this cell Li goes to Li+ spontaneously and Cu2+ goes to Cu spontaneously.
Finally, a positive value of E° for a half cell reaction in a standard table of E° values does not indicate that it is a spontaneous reaction, in as much as a negative value of E° for a half cell reaction in that table of E° values does not indicate a nonspontaneous reaction.
A half cell reaction does not occur by itself. So, when combined with another half cell reaction, a cell is formed and the two half cell reactions occur spontaneously (as you guessed correctly) to give a spontaneous cell reaction with a value of E° for the cell which is the difference between the two E°s of the individual half cells chosen.
P. Radhakrishnamurty